Abstract
Scoparone, which is a major constituent of Artemisia capillaries, has been identified as an anticoagulant, hypolipidemic, vasorelaxant, anti-oxidant and anti-inflammatory drug, and it is used for the traditional treatment of neonatal jaundice. Therefore, we hypothesized that scoparone could suppress the proliferation of VSMCs by interfering with STAT3 signaling. We found that the proliferation of these cells was significantly attenuated by scoparone in a dose-dependent manner. Scoparone markedly reduced the serum-stimulated accumulation of cells in the S phase and concomitantly increased the proportion of cells in the G0/G1 phase, which was consistent with the reduced expression of cyclin D1, phosphorylated Rb and survivin in the VSMCs. Cell adhesion markers, such as MCP-1 and ICAM-1, were significantly reduced by scoparone. Interestingly, this compound attenuated the increase in cyclin D promoter activity by inhibiting the activities of both the WT and active forms of STAT3. Similarly, the expression of a cell proliferation marker induced by PDGF was decreased by scoparone with no change in the phosphorylation of JAK2 or Src. On the basis of the immunofluorescence staining results, STAT3 proteins phosphorylated by PDGF were predominantly localized to the nucleus and were markedly reduced in the scoparone-treated cells. In summary, scoparone blocks the accumulation of STAT3 transported from the cytosol to the nucleus, leading to the suppression of VSMC proliferation through G1 phase arrest and the inhibition of Rb phosphorylation. This activity occurs independent of the form of STAT3 and upstream of kinases, such as Jak and Src, which are correlated with abnormal vascular remodeling due to the presence of an excess of growth factors following vascular injury. These data provide convincing evidence that scoparone may be a new preventative agent for the treatment of cardiovascular diseases.
Introduction
Myocardial infarction is significantly increased in patients with complicated cardiovascular diseases, such as atherosclerosis, diabetes, hypertension, stroke and coronary artery disease.1 Balloon angioplasty, which is a technique that includes the mechanical widening of narrowed or obstructed arteries, has been used to treat stenotic coronary arteries and atherosclerosis in narrow blood vessels. Although novel clinical approaches for the prevention of restenosis using drug-eluting stents are available and in use, the mechanisms involved are not fully understood.2 Vascular remodeling has been studied extensively as a critical mechanism that leads to cardiovascular diseases as a result of vascular damage.
Many studies in the literature have suggested that growth factors and cytokines, such as platelet-derived growth factor (PDGF), have major roles in the pathogenesis of restenosis.3 Although the expression of PDGF and its receptors are very low or undetectable in normal vessels, PDGF expression is increased at vascular injury sites, followed by the increased activation of platelets and the recruitment of monocytes.4 The expression of exogenous PDGF in the arteries can induce intimal thickening through the stimulation of VSMC proliferation and migration.5 It is noteworthy that the inhibition of PDGF and the PDGF receptor by immunological, molecular, biological and pharmacological methods can prevent the development of restenotic lesions in vessels in vivo.4 Furthermore, signal transducer and activator of transcription 3 (STAT3), which is one of major downstream mediators of PDGF signaling, is activated in these vessels.6
STAT3 is a member of the STAT family of transcription factors, of which there are seven that regulate various cytokines, growth factors and hormones.7 The binding of cytokines and growth factors, such as interleukin (IL)-6 and PDGF, to their cognate cell surface receptors, such as gp130 and PDGF receptor, triggers the activation of receptor tyrosine kinases or non-receptor tyrosine kinases, such as JAK2 or Src.8, 9 These upstream kinases in turn can activate STAT3 by phosphorylating specific tyrosine (Y705) and serine 727 residues (S727).10 Once they are phosphorylated, STAT3 homo-dimers in the nucleus regulate target genes, such as cell adhesion molecules (for example, matrix metallopeptidase (MMP)-9).11 Therefore, STAT3 can mediate the effects of various cytokines, especially PDGF, in the vascular remodeling that follows vascular damage.
Scoparone (6,7-dimethoxycoumarin), which is a major constituent of the Chinese herb Artemisia capillaries, has been identified to be a potent anti-coagulant, vasorelaxant, anti-oxidant, hypolipidemic and anti-inflammatory agent.12, 13, 14, 15, 16 The protective effect of scoparone involves anti-inflammatory functions that occur via the inhibition of NF-κB activity, leading to the downregulation of pro-inflammatory cytokines and chemokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8 and monocyte chemoattractant protein (MCP)-1.16 Interestingly, the free radical scavenging property of scoparone leads to the attenuation of intima thickening and improves hemodynamics in cholesterol-fed alloxan-diabetic rabbits concurrent with decreases in plasma lipid and lipoprotein cholesterol concentrations.17 However, the molecular targets of scoparone and its ability to halt restenosis have been poorly investigated. In this study, we demonstrated that scoparone can suppress VSMC proliferation, and we provide the molecular mechanism of action related to the direct inhibition of STAT3 activity.
Materials and Methods
Animal and primary VSMC cultures
The procedures used in this study were approved by the Animal Care and Use Committee of the Kyungpook National University School of Medicine and conducted according to institutional guidelines. VSMCs were isolated from the thoracic aortas of 4-week-old Sprague–Dawley (SD) rats (Hyochang, Daegu, Korea) anesthetized with pentobarbital sodium anesthesia (50 mg kg−1, i.p.) and were maintained in DMEM (high glucose; 4.5 g l−1) supplemented with 10% FBS at 37 °C in a humidified 5% CO2 incubator. Cells from the third to fifth passages were used for the experiments, which were performed as described previously.18 The purity of the VSMCs was determined by positive staining with a monoclonal antibody to smooth muscle-specific α-actin (catalog #A5228, Sigma, St Louis, MO, USA). All animals were provided ad libitum access to food and water before the study.
Cell migration assay
The rat VSMCs were seeded in six-well plates at 90% confluence and were starved in serum-free media for 24 h followed by incubation in 1% FBS for an additional 24 h. The cells in the wells were detached by razor scraping, rinsed with PBS, stimulated in media containing 20% FBS, co-treated with 0.2% DMSO or 500 μM scoparone for 24 h and then stained with hematoxylin. Cell images were obtained via microscopy with a × 10 objective.
Cell proliferation assays
For the cell proliferation assay, VSMCs (1.5 × 103) were seeded in 96-well plates and serum-starved for 48 h. After starvation, the cells were stimulated with 20% FBS and co-treated with 0.2% DMSO or 500 μM scoparone in 0.2% DMSO for 72 h using a WST-1 kit (Dojindo Laboratories, Kumamoto, Japan). Data (arbitrary fluorescence units; AFUs) were presented as fold changes vs the control.
Transient transfection assay of HepG2 cells
HepG2 cells were cultured in MEM supplemented with 10% FBS and antibiotics. For transient expression of the plasmids, HepG2 (8 × 104) cells were seeded in 24-well plates, and, on the next day, they were transfected with 100–200 ng of plasmid using TransIT-LT1 transfection reagent (Mirus Bio Incorporation, Madison, WI, USA). Cytomegalovirus (CMV)-β-galactosidase plasmids were co-transfected as an internal control. After 48 h, the cells were harvested for luciferase and β-galactosidase assays. Luciferase activity was normalized to β-galactosidase activity.
FACS analysis
To examine the cell cycle, VSMCs (3 × 105) were seeded in 100-mm culture plates. The cells were synchronized at the G0/G1 phase by serum starvation for 72 h, stimulated with 20% FBS and treated with 0.2% DMSO or 500 μM scoparone for 20 h. They were then trypsinized and washed once with cold PBS containing 0.01% CaCl2 and 2% FBS. Next, they were fixed in 95% cold ethanol at −20 °C for 1 h, stained with 1 ml of staining solution (40 μg ml−1 propidium iodide, 10 μg ml−1 RNase A and 0.1% NP-40) for 1 h in the dark at 4 °C and analyzed with a FACSCalibur system (BD Bioscience, San Jose, CA, USA).
Immunofluorescence analysis
VSMCs were seeded onto glass slides and allowed to attach for 24 h and then treated with scoparone for 4 h. The cells were fixed with 95% ethanol for 15 min at −20 °C and incubated with antibodies against pSTAT3 (Y705) (catalog #9138, Cell Signaling, Beverley, MA, USA) or STAT3 (catalog #9132, Cell Signaling) followed by incubation with Alexa-488 or Alexa-568-labeled secondary antibodies, respectively. Cell nuclei were stained with DAPI, and confocal images were obtained and merged.
Reagents and plasmids
Scoparone (6,7-dimethoxycoumarin, catalog #254886) and AG490 (JAK2 inhibitor, catalog #T3434) were purchased from Sigma Aldrich (St Louis, MO, USA). PDGF (catalog #120-HD-005) was obtained from R&D Systems (McKinley Place, Minneapolis, MN, USA). Cyclin-pro-Luc (−985/+281) and constructs expressing wild-type STAT3-flag or the constitutively active form of STAT3 (STAT3C-FLAG) were kind gifts of Dr James E. Darnell (the Rockefeller University, New York, NY, USA).
Western blot analysis
Cell lysates were prepared using a lysis buffer (20 mM Tris, pH7.4, 10 mM Na4P2OH, 100 mM NaF, 2 mM Na3VO4, 5 mM EDTA, pH 8.0, 0.1 mM PMSF and 1% NP-40) containing proteinase and phosphatase inhibitors. Proteins were resolved by Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to an Immobilon-P membrane (Millipore, Billerica, MA, USA). After blocking, the membrane was incubated with primary antibodies. Anti-retinoblastoma protein (pRb) (catalog #sc-50) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-pRb (catalog #9308), cyclin D1 (catalog #2978) and anti-cyclin E (catalog #34189) antibodies were obtained from Cell Signaling, and an anti-β-actin (Catalog #A5441) antibody was purchased from Sigma. The membrane was washed and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody, and signals were detected using an ECL western blotting detection system.
Statistical analysis
Numerical data are presented as the mean±s.d. Statistical analyses were performed using an unpaired Student's t-test or one-way analysis of variance (ANOVA) as appropriate, and a P-value of <0.05 was considered statistically significant.
Results
Scoparone inhibits serum-stimulated migration and proliferation of VSMCs
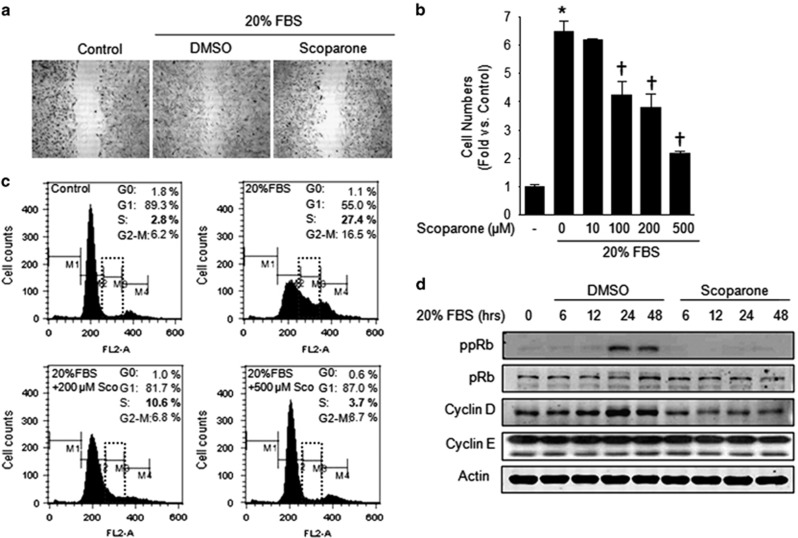
To identify the effect of scoparone on the migration of VSMCs, we performed a cell migration assay in which cells were stimulated with 20% FBS in the presence of 200 μM scoparone after the scraping of quiescent cells. As expected, the FBS-induced migration of the scraped quiescent cells was attenuated by scoparone (Figure 1a). Consistent with the degree of migration inhibition by scoparone, the serum-stimulated proliferation of the VSMCs was significantly reduced by this compound in a dose-dependent manner, reaching a 66±2% reduction at 500 μM of scoparone (Figure 1b). Scoparone did not alter the proliferation of VSMCs that were not stimulated with serum, suggesting that it had cytostatic, and not cytotoxic, effects on these cells (data not shown). To determine which phase of the cell cycle was regulated by scoparone and whether apoptosis was involved in its antiproliferative effects, we performed FACS cell cycle analysis. Quiescent VSMCs synchronized at G0/G1 phase (89.3→55.0%) showed marked transitions to S phase (2.8→27.4%) and G2-M phase (6.2→16.5%) following serum stimulation (Figure 1c). However, scoparone strongly reduced the serum-stimulated accumulation of cells at S phase by 87% and concomitantly increased the percentage of cells at G0/G1 phase by 58% in a dose-dependent manner (Figure 1c). In particular, scoparone did not lead to any significant accumulation of cells at sub-G1 phase, indicating that apoptosis was not involved in its anti-proliferative effect on the VSMCs. Next, we investigated its effect on the expression of cell regulatory proteins, including cyclin D1, and the phosphorylation of pRb (ppRb), which are required for cell cycle progression from the G0/G1 to S phases. On the basis of western blot analysis, the levels of cyclin D1 and ppRb were markedly increased between 24 and 48 h after serum stimulation, but scoparone significantly diminished ppRb in time- and dose-dependent manners (Figure 1d). These results suggest that scoparone suppresses VSMC proliferation by G1 cell cycle arrest through inhibiting ppRb and cyclin D protein expression.
Figure 1.
Scoparone effects on vascular smooth muscle cell (VSMC) migration and the cell cycle. (a) Cell migration assay. Quiescent VSMCs were treated with 20% FBS±scoparone (200 μM) for 24 h, and VSMCs without FBS treatment were used as a control. (b) WST-1 cell proliferation assay. The proliferation rate of the VSMCs was determined according to the numbers of cells induced by 20% FBS with different doses of scoparone for 72 h compared with the numbers of quiescent cells. *P<0.05 vs FBS-free media and †P<0.05 vs 20% FBS stimulation. (c) Fluorescence-activated cell sorting (FACS) analysis. For cell cycle determination, VSMCs were starved for FBS for 72 h and then incubated with 20% FBS±scoparone (0–500 μM) for 20 h and sorted into the G0, G1, S and G2-M phases by FACS. (d) Western blot analysis. Quiescent cells were stimulated by 20% FBS±scoparone (500 μM) in a time-dependent manner for 48 h. Cell-cycle-regulatory proteins, including retinoblastoma protein (pRb), cyclin D, cyclin E and actin as a control, were visualized in representative immunoblots. ppRb indicates phosphorylated pRb.
Scoparone downregulates genes, including those encoding cell cycle proteins and proliferation markers, at the transcriptional level
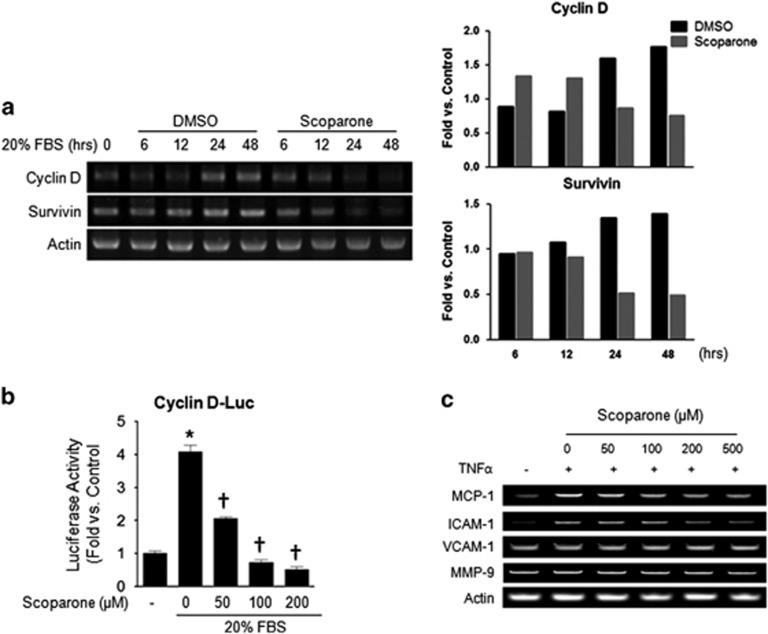
Next, we examined the effects of scoparone on the mRNA expression of cyclin D1 and survivin, which have important roles in VSMC proliferation as STAT3 downstream genes.19, 20 Semi-quantitative RT–PCR analysis showed that scoparone markedly attenuated the serum-stimulated mRNA expression of cyclin D1 and survivin at 24 and 48 h (Figure 2a). In addition, we assessed the inhibitory effect of scoparone on cyclin D1 promoter activity by a luciferase assay and found that it suppressed this activity (Figure 2b). We did not find any differences in cyclin E levels (data not shown). Similarly, the upregulation of chemokines, such as MCP-1 and intercellular adhesion molecule (ICAM)-1, by TNF-α appeared to be attenuated by scoparone (Figure 2c). The quantifications of MCP1, ICAM-1, VCAM-1 and MMP9 were shown in Supplementary Figure 1. In addition, scoparone significantly decreased the promoter activity of NF-κB in a dose-dependent manner (data not shown). These data demonstrate that the inhibitory effect of scoparone on proliferation and migration might be due to interference with the activities of transcription factors.
Figure 2.
Transcriptional regulation by scoparone in vascular smooth muscle cells (VSMCs) or HepG2 cells. (a) RT–PCR. The mRNA expression levels of cyclin D, survivin D and actin as a control are shown in representative agarose gels. Total RNA was isolated from VSMCs after incubation with 20% FBS±scoparone (200 μM) for different durations (0–48 h). (b) Promoter activity assay by luciferase reporter assay in HepG2 cells. Cells were incubated with 20% FBS±scoparone (0–200 μM) for 24 h after cyclin D promoter region-luciferase transfection. Relative luciferase activities were determined and normalized according to the activities of the control cells. (c) Semi-quantitative PCR was performed to assess the expression of chemokines and cell adhesion molecules, such as MCP-1, ICAM-1, VCAM-1, MMP-9 and actin as a control. VSMCs were treated with TNF-α (10 ng ml−1) under different scoparone concentrations (0–500 μM) for 24 h. *P<0.05 vs control and †P<0.05 vs 20% FBS (treated or TNF-α only).
Scoparone suppresses transcriptional activity of STAT
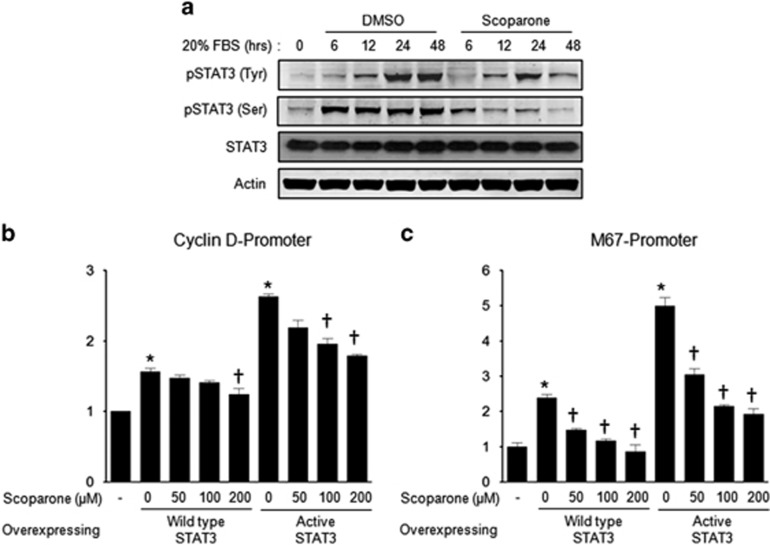
Several lines of evidence suggest that the JAK-STAT pathway has a major role in vascular proliferation and migration.21, 22 To determine whether the transcriptional factors involved in vascular remodeling are regulated by scoparone, we examined its effects on the phosphorylated forms of STAT3 after serum activation. The phosphorylation of both the Tyr705 and Ser727 residues of STAT3 was increased in a time-dependent manner (Figure 3a), and scoparone reduced Tyr705 and Ser727 phoshorylation at each time point. We further confirmed its inhibitory effect on STAT3 activity in HepG2 cells harboring the wild-type (WT) or constitutively active (CA) form of STAT3. We also evaluated the activities of the cyclin-D and M67 promoters, which were found to be regulated by the cytokine PDGF-BB and mediated by STAT activity,23 by measuring luciferase activity (Figures 3b and c). Scoparone (200 μM) completely blocked the stimulation of M67 promoter activity due to the overexpression of both STAT forms (WT and CA) (Figure 3c). We found that the transcriptional activities of both the basal and constitutively active forms of STAT3 were significantly inhibited by scoparone.
Figure 3.
Regulation of STAT3 activity by scoparone in vascular smooth muscle cells (VSMCs) or HepG2 cells. (a) Western blot analysis of phosphorylated STAT(Tyr705) and STAT(Ser727). Total proteins were isolated from VSMCs treated with 20% FBS±scoparone (200 μM) for 0–48 h, and the band for each protein was visualized in representative blots. (b, c) Promoter activity assay by luciferase reporter gene. The quantification of STAT activity was performed by assessing the activities of the cyclin-D (b) and M67 promoters (c). HepG2 cells were co-transfected with the wild type or constitutively active form of STAT and the above-mentioned plasmids, respectively, for 24 h. *P<0.05 vs no STAT overexpression and †P<0.05 vs no scoparone treatment.
Scoparone reduces PDGF-BB-stimulated expression of cyclin D1 and pRb proteins by suppressing STAT3 activity
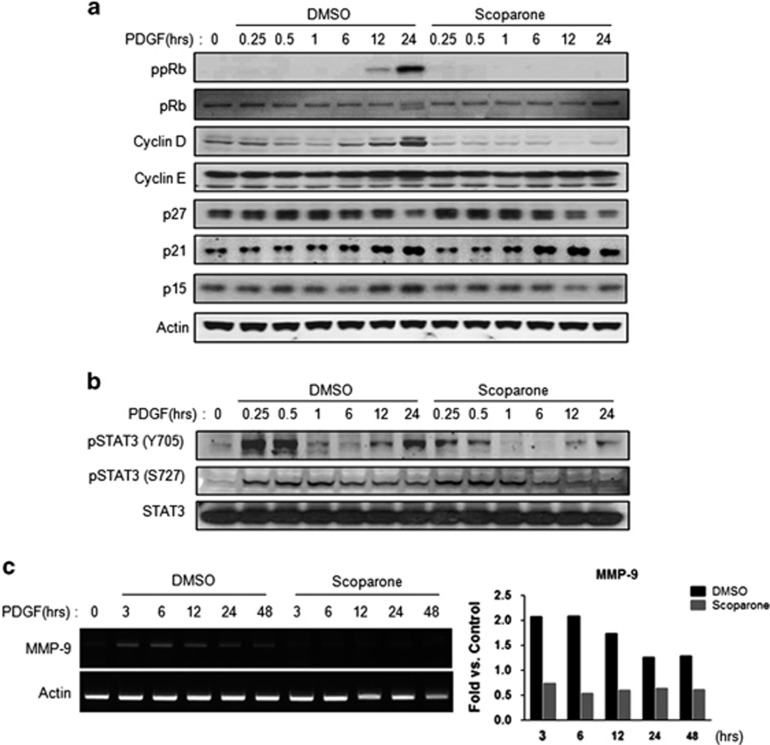
Because serum contains various factors that can affect VSMC proliferation, we focused on the effect of scoparone on VSMC proliferation following stimulation with PDGF, which has been established as one of the cytokines that is upregulated after vascular injury.4 Similar to what was observed following serum stimulation, PDGF-BB increased phosphorylated pRb and cyclin D1 levels at 24 h, and these levels were markedly attenuated by scoparone (Figure 4a). Concomitantly, scoparone diminished the PDGF-induced phosphorylation of STAT3 (Y705), which resulted from PDGF-BB-induced decreases in MMP-9 mRNA at earlier time points (Figures 4b and c). These data suggest that the inhibitory effect of scoparone on the proliferation of VSMCs is mainly mediated by inference with the transcriptional activity of Jak/STAT signaling activated by cytokines and growth factors during vascular remodeling.
Figure 4.
Inhibitory effects of scoparone on STAT signaling induced by PDGF in vascular smooth muscle cells (VSMCs). (a, b) Western blot analysis of cell regulatory proteins (a) and different forms of STAT (b) treated with 20 ng ml−1 PDGF±200 μM scoparone for different durations. Total proteins were isolated from VSMCs induced at these differing time points (0–24 h). ppRb indicates phosphorylated retinoblastoma protein (pRb). (c) RT–PCR. Total RNA was isolated from VSMCs incubated with 20 ng ml−1 PDGF±200 μM scoparone for different durations (0–48 h). The mRNA expression levels of MMP-9 and actin as a control were visualized in representative agarose gels.
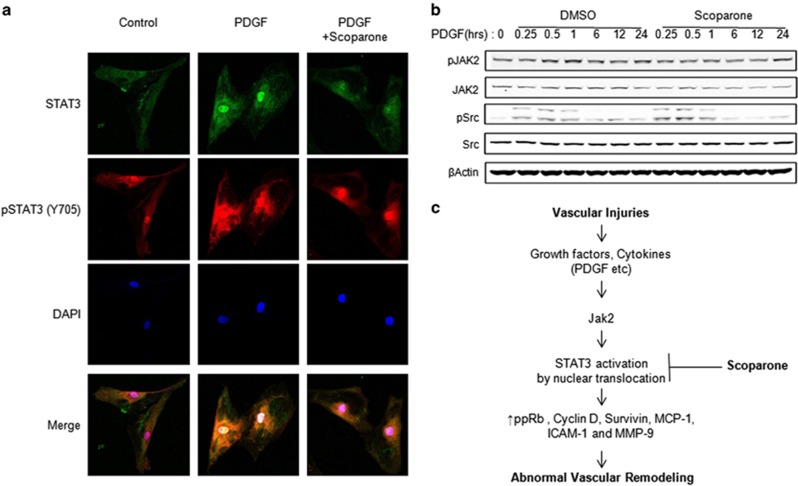
Scoparone prevents nuclear accumulation of STAT3 after PDGF induction
Previously, it has been reported that STAT3 translocation from the cytosol to the nucleus by PDGF induction is essential for its transcriptional activity, and this finding was confirmed by biochemical analysis6. Similarly, our immunofluorescence data showed that STAT3 proteins were predominantly translocated to the nucleus from the cytosol following PDGF-BB induction (Figure 5a and Supplementary Figure 2). To determine whether the inhibitory effect of scoparone on STAT3 phosphorylation is due to the suppression of its upstream signaling, we assessed the effects of scoparone on the phosphorylation of JAK2 and Src, which are two major upstream STAT3 kinases. Scoparone did not reduce the protein levels of constitutively phosphorylated JAK2. Furthermore, Src phosphorylation was not inhibited but was actually increased by scoparone (Figure 5b). Collectively, these observations suggest that this compound inhibits the phosphorylation and nuclear accumulation of STAT3 independent of the inhibition of the upstream kinases JAK2 and Src. The nuclear pSTAT3 signal was markedly reduced in the scoparone-treated cells. Taken together, these findings indicate that scoparone inhibits constitutive and PDGF-BB-stimulated phosphorylation as well as the nuclear accumulation of pSTAT3 in VSMCs (Figure 5c).
Figure 5.
STAT3 regulation by PDGF induction in vascular smooth muscle cells (VSMCs). (a) STAT3 localization by immunofluorescence microscopy. VSMCs were induced by 20 ng ml−1 PDGF for 30 min±pre-incubation with 500 μM scoparone for 4 h and were processed for immunofluorescence staining using antibodies against pSTAT3 (Y705) or STAT3. Cell nuclei were stained with DAPI, and confocal images were obtained and merged. (b) Western blot analysis of Jak2 and Src protein expression. (c) Proposed scheme representing that scoparone can inhibit VSMC proliferation by inhibiting STAT 3 activation mediated by a growth factor. Total proteins were isolated from VSMCs induced by 20 ng ml−1 PDGF±200 μM scoparone for different durations (0–24 h), and the total proteins were used for western blot analysis. β-Actin was used as a loading control.
Discussion
VMSC proliferation is one of the key mechanisms involved in the development and progression of neointimal hyperplasia, which contributes to the pathogenesis of atherosclerosis and restenosis.7 There have been many experimental trials aimed at developing agents that effectively decrease the neointimal proliferation of vessels after injury and identifying the mechanisms that may be involved. The thrombin receptor antagonist atopaxar and the cyclin-dependent kinase inhibitor indirubin-3′-monoxime can block the abnormal proliferation of VSMCs in a model of vascular injury or by G0/G1 arrest during PDGF-induced VSMC proliferation, respectively.24, 25 In the present study, we showed that scoparone, which is a derivative of the Chinese herb Artemisia capillaries, potently inhibited DNA synthesis induced by growth factors in VSMCs via the interruption of STAT3 activity, which led to the arrest of cells in the G1 phase of the cell cycle. In addition, we found that enhanced STAT3 translocation to the nucleus by PDGF was significantly attenuated independent of the regulation of upstream Jak2 or Src signaling in VSMCs.
As mentioned earlier, PDGF is a potent cytokine that can regulate abnormal vascular remodeling during motility and the process of wound healing that is mediated by JAK2-STAT3 signaling.23 Growth hormone-mediated JAK2-STAT signaling can be attenuated by the dephosphorylation of tyrosine residues located in its receptors with protein tyrosine phosphatase 1B. This is essential for limiting the actions of growth hormones during cellular stress or with a tyrosine kinase inhibitor,26, 27 while the increased intake of dietary flavonoids, such as chrysin, can attenuate PDGF-induced proliferation by restoring protein tyrosine phosphatase activity.28 Upregulated STAT3 associated with the GTPase Rac1 can translocate to the nucleus after PDGF treatment, resulting in the proliferation of airway smooth muscle cells, which may represent a therapeutic target in patients with asthma.6 Similarly, JAK2/STAT3 inhibition by thrombopoietin mimetics can attenuate myocardial post-ischemic injury and the proliferation of mesangial cells.29, 30, 31 The blockade of STAT3 activity during stress might alleviate these pathological symptoms.
Recently, we have reported that the inhibition of STAT3 activity, which is the direct target of scoparone, leads to anti-tumor activity in DU-145 prostate cancer cells.32 Interestingly, scoparone can attenuate the proliferation of cancer cells that display heightened STAT3 activity. In addition, the inhibition of STAT3 activity by scoparone has a critical role in the proliferation of VSMCs based on our data. Further, the beneficial effects of this compound have been demonstrated in patients with hepatic failure through the inhibition of Toll-like receptors,33 in lipopolysaccharide-activated human endothelial cells through decreases in tissue factor expression,15 and in RINm5F cells by conferring protection against IL-1- and IFN-mediated cytotoxicity.34 In addition, we observed that scoparone inhibits platelet aggregation, which might be related to interference with the binding of p21-activated kinases and the Rho family of small GTP binding proteins, which is consistent with the findings of Aslan et al.35 Taken together, these findings suggest that scoparone, which has been traditionally used for the treatment of metabolic diseases, prohibits abnormal cell proliferation by attenuating STAT3 activity, leading to G0/G1 arrest and reductions in vasoactive molecules. In addition to the interference of scoparone with STAT3 activity during VSMC proliferation, the upregulation of chemokines, such as MCP-1, ICAM-1 and TNF-α, might also be caused by this compound, resulting in protection from cardiovascular diseases. This finding should be further investigated. Therefore, scoparone could be a therapeutic drug for the treatment of patients with vascular diseases.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2012R1A2A1A03670452 to IK Lee), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and the Ministry of Health & Welfare, Republic of Korea (A111345 to IK Lee), and the Medical Cluster R&D Support Project of the Daegu Gyeongbuk Medical Innovation Foundation, Republic of Korea (2013) (to S Park). We thank Assistant Professor Keisa W. Mathis at the Department of Integrative Physiology, University of North Texas Health Science Center, Fort Worth, TX, USA and Professor Jiyoong Hong at the Department of Chemistry, Duke University, Durham, NC, USA for their generous reviews and discussions of this article.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
Supplementary Material
References
- Rogers WJ, Canto JG, Lambrew CT, Tiefenbrunn AJ, Kinkaid B, Shoultz DA, et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: the National Registry of Myocardial Infarction 1, 2 and 3. J Am Coll Cardiol. 2000;36:2056–2063. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- Kraitzer A, Kloog Y, Zilberman M. Approaches for prevention of restenosis. J Biomed Mater Res B Appl Biomater. 2008;85:583–603. doi: 10.1002/jbm.b.30974. [DOI] [PubMed] [Google Scholar]
- Levitzki A. PDGF receptor kinase inhibitors for the treatment of restenosis. Cardiovasc Res. 2005;65:581–586. doi: 10.1016/j.cardiores.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Pompili VJ, Gordon D, San H, Yang Z, Muller DW, Nabel GJ, et al. Expression and function of a recombinant PDGF B gene in porcine arteries. Arterioscler Thromb Vasc Biol. 1995;15:2254–2264. doi: 10.1161/01.atv.15.12.2254. [DOI] [PubMed] [Google Scholar]
- Simeone-Penney MC, Severgnini M, Rozo L, Takahashi S, Cochran BH, Simon AR. PDGF-induced human airway smooth muscle cell proliferation requires STAT3 and the small GTPase Rac1. Am J Physiol Lung Cell Mol Physiol. 2008;294:L698–L704. doi: 10.1152/ajplung.00529.2007. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Bromberg J, Darnell JE., Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- Song Y, Qian L, Song S, Chen L, Zhang Y, Yuan G, et al. Fra-1 and Stat3 synergistically regulate activation of human MMP-9 gene. Mol Immunol. 2008;45:137–143. doi: 10.1016/j.molimm.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Huang HC, Chu SH, Chao PD. Vasorelaxants from Chinese herbs, emodin and scoparone, possess immunosuppressive properties. Eur J Pharmacol. 1991;198:211–213. doi: 10.1016/0014-2999(91)90624-y. [DOI] [PubMed] [Google Scholar]
- Huang HC, Weng YI, Lee CR, Jan TR, Chen YL, Lee YT. Protection by scoparone against the alterations of plasma lipoproteins, vascular morphology and vascular reactivity in hyperlipidaemic diabetic rabbit. Br J Pharmacol. 1993;110:1508–1514. doi: 10.1111/j.1476-5381.1993.tb13993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult JR, Paya M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol. 1996;27:713–722. doi: 10.1016/0306-3623(95)02112-4. [DOI] [PubMed] [Google Scholar]
- Lee YM, Hsiao G, Chang JW, Sheu JR, Yen MH. Scoparone inhibits tissue factor expression in lipopolysaccharide-activated human umbilical vein endothelial cells. J Biomed Sci. 2003;10:518–525. doi: 10.1007/BF02256113. [DOI] [PubMed] [Google Scholar]
- Jang SI, Kim YJ, Kim HJ, Lee JC, Kim HY, Kim YC, et al. Scoparone inhibits PMA-induced IL-8 and MCP-1 production through suppression of NF-kappaB activation in U937 cells. Life Sci. 2006;78:2937–2943. doi: 10.1016/j.lfs.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Huang JC, Wang XR, Sun AX, Wang RX. [Effects of scoparone on hemodynamics in anesthetized rabbits] Zhongguo Yao Li Xue Bao. 1993;14 (Suppl:S18–S21. [PubMed] [Google Scholar]
- Komalavilas P, Shah PK, Jo H, Lincoln TM. Activation of mitogen-activated protein kinase pathways by cyclic GMP and cyclic GMP-dependent protein kinase in contractile vascular smooth muscle cells. J Biol Chem. 1999;274:34301–34309. doi: 10.1074/jbc.274.48.34301. [DOI] [PubMed] [Google Scholar]
- Zhao M, Jiang B, Gao FH. Small molecule inhibitors of STAT3 for cancer therapy. Curr Med Chem. 2011;18:4012–4018. doi: 10.2174/092986711796957284. [DOI] [PubMed] [Google Scholar]
- Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AR, Takahashi S, Severgnini M, Fanburg BL, Cochran BH. Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1296–L1304. doi: 10.1152/ajplung.00315.2001. [DOI] [PubMed] [Google Scholar]
- Banes AK, Shaw SM, Tawfik A, Patel BP, Ogbi S, Fulton D, et al. Activation of the JAK/STAT pathway in vascular smooth muscle by serotonin. Am J Physiol Cell Physiol. 2005;288:C805–C812. doi: 10.1152/ajpcell.00385.2004. [DOI] [PubMed] [Google Scholar]
- Neeli I, Liu Z, Dronadula N, Ma ZA, Rao GN. An essential role of the Jak-2/STAT-3/cytosolic phospholipase A(2) axis in platelet-derived growth factor BB-induced vascular smooth muscle cell motility. J Biol Chem. 2004;279:46122–46128. doi: 10.1074/jbc.M406922200. [DOI] [PubMed] [Google Scholar]
- Schwaiberger AV, Heiss EH, Cabaravdic M, Oberan T, Zaujec J, Schachner D, et al. Indirubin-3'-monoxime blocks vascular smooth muscle cell proliferation by inhibition of signal transducer and activator of transcription 3 signaling and reduces neointima formation in vivo. Arterioscler Thromb Vasc Biol. 2010;30:2475–2481. doi: 10.1161/ATVBAHA.110.212654. [DOI] [PubMed] [Google Scholar]
- Kogushi M, Matsuoka T, Kuramochi H, Murakami K, Kawata T, Kimura A, et al. Oral administration of the thrombin receptor antagonist E5555 (atopaxar) attenuates intimal thickening following balloon injury in rats. Eur J Pharmacol. 2011;666:158–164. doi: 10.1016/j.ejphar.2011.05.034. [DOI] [PubMed] [Google Scholar]
- Chen Z, Lee FY, Bhalla KN, Wu J. Potent inhibition of platelet-derived growth factor-induced responses in vascular smooth muscle cells by BMS-354825 (dasatinib) Mol Pharmacol. 2006;69:1527–1533. doi: 10.1124/mol.105.020172. [DOI] [PubMed] [Google Scholar]
- Gu F, Dube N, Kim JW, Cheng A, Ibarra-Sanchez Mde J, Tremblay ML, et al. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol. 2003;23:3753–3762. doi: 10.1128/MCB.23.11.3753-3762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HM, Wu MW, Pan SL, Peng CY, Wu PH, Wu WB. Chrysin restores PDGF-induced inhibition on protein tyrosine phosphatase and reduces PDGF signaling in cultured VSMCs. J Nutr Biochem. 2012;23:667–678. doi: 10.1016/j.jnutbio.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Wang T, Mao X, Li H, Qiao S, Xu A, Wang J, et al. N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic Biol Med. 2013;63:291–303. doi: 10.1016/j.freeradbiomed.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Chan KY, Zhou L, Xiang P, Li K, Ng PC, Wang CC, et al. Thrombopoietin improved ventricular function and regulated remodeling genes in a rat model of myocardial infarction. Int J Cardiol. 2013;167:2546–2554. doi: 10.1016/j.ijcard.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Hirai T, Masaki T, Kuratsune M, Yorioka N, Kohno N. PDGF receptor tyrosine kinase inhibitor suppresses mesangial cell proliferation involving STAT3 activation. Clin Exp Immunol. 2006;144:353–361. doi: 10.1111/j.1365-2249.2006.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Kim JY, Kim HJ, Park KG, Harris RA, Cho WJ, et al. Scoparone exerts anti-tumor activity against DU145 prostate cancer cells via inhibition of STAT3 activity. PLoS One. 2013;8:e80391. doi: 10.1371/journal.pone.0080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JW, Kim DW, Choi JS, Kim YS, Lee SM. Scoparone attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure through inhibition of toll-like receptor 4 signaling in mice. Food Chem Toxicol. 2013;57:132–139. doi: 10.1016/j.fct.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Kim EK, Kwon KB, Lee JH, Park BH, Park JW, Lee HK, et al. Inhibition of cytokine-mediated nitric oxide synthase expression in rat insulinoma cells by scoparone. Biol Pharm Bull. 2007;30:242–246. doi: 10.1248/bpb.30.242. [DOI] [PubMed] [Google Scholar]
- Aslan JE, Itakura A, Haley KM, Tormoen GW, Loren CP, Baker SM, et al. p21 activated kinase signaling coordinates glycoprotein receptor VI-mediated platelet aggregation, lamellipodia formation, and aggregate stability under shear. Arterioscler Thromb Vasc Biol. 2013;33:1544–1551. doi: 10.1161/ATVBAHA.112.301165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.