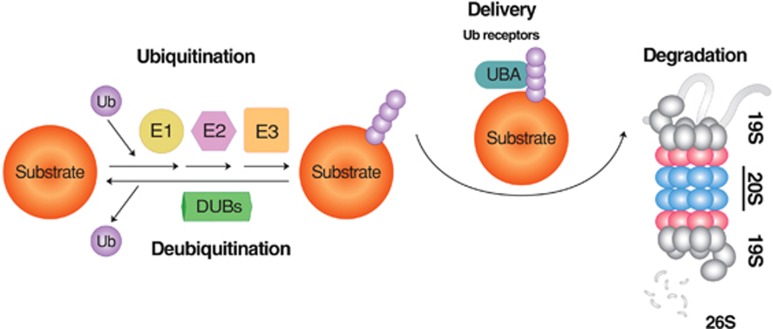
Figure 1.
The degradation of short-lived proteins by the UPS. In this selective proteolytic system, Ub is first activated by E1 and subsequently transferred to E2. In parallel, misfolded substrates of the UPS are recognized by molecular chaperones, such as CHIP, and associated with Ub ligases that promote the transfer of E2-conjugated Ub to specific Lys residues of substrates. Ubiquitinated substrates are deubiquitinated, unfolded, fed into the narrow chamber of the proteasome, and progressively cleaved into small peptides. Depending on the types of E3 ligases, Ub can be directly transferred from E2 to the substrate or via a two-step process that involves a transient binding of E3 to Ub. The repetition of this reaction results in the growth of a singly conjugated Ub to a chain of Ub with different topologies, depending on how Ub is conjugated to another Ub. Modified from Wang and Robbins.226