Figure 1.
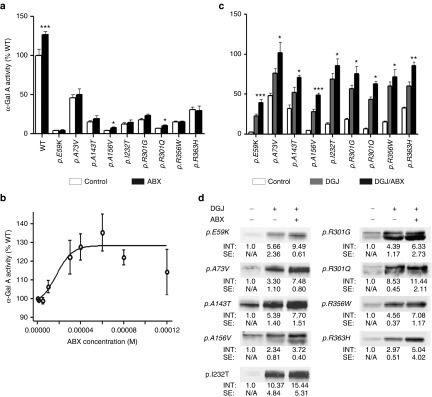
Effect of ABX on overexpressed mutant forms of α-Gal A in HEK-293H cells. ABX was administered 6 hours after transfection of the GLA cDNA-containing plasmids and then cultured for 60 hours changing the media any other day adding fresh treatment as described in the Materials and Methods section (a) Bona fide analysis with 40 µmol/l ABX revealed a tendency to mildly increase intracellular activities of several mutant α-Gal A forms that was significant for p.A156V and p.R301Q. Wild-type enzyme increased markedly upon the addition of the compound as well. (b) Concentration–response relation analysis showed increasing wild-type α-Gal A activity. An EC50 of 17.4 µmol/l was calculated by a nonlinear regression analysis A maximum stimulatory effect was obtained at 60 µmol/l. At concentrations ≥80 µmol/l, the α-Gal A activity dropped back to normal. (c) In the same culture system as under (a), the GLA expressing HEK-293H cells were DGJ or DGJ/ABX combination-treated. The DGJ-responsive α-Gal A forms (see also Supplementary Figure S1) displayed considerable gains from the additional administration of ABX. (d) Western blot of the mutant α-Gal A forms indicated higher levels of intracellular enzyme after treatment with DGJ in combination with 40 µmol/l ABX compared to the monotherapy. Western blots were repeated at least three times. In each lane 30 µg of total protein was loaded and separated by SDS-PAGE. Semiquantitative analysis was carried out using the Odyssey software v1.2. Calculated intensities were normalized for GAPDH internal loading control (not shown). The average intensities (“INT”) are given as fold change ± standard error. Enzyme activity values are shown as mean ± SEM (n ≥ 5). Results were considered significant if *P < 0.05, **P < 0.01, ***P < 0.005. Control treatment denotes the respective carrier solvent used for the compounds (DGJ was diluted in hypure H2O as a 10 mmol/l stock solution, ABX was typically diluted in DMSO (100 mmol/l).
