Fig. 2.
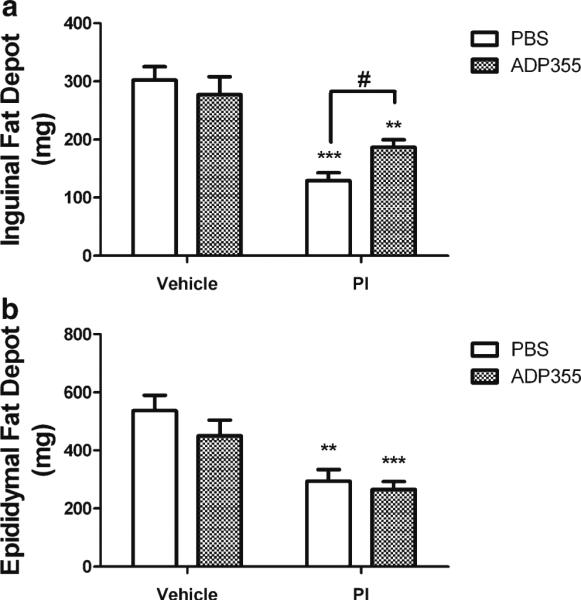
ADP355 preserves subcutaneous, but not visceral fat depots in PI-treated mice. Male C57BL/6 mice were given daily administration of 10 % ethanol/15 % propylene glycol (vehicle; left bars) or 150 mg lopinavir/37.5 mg ritonavir/kg (PI; right bars) for 28 days. Additionally, randomly selected vehicle (open bars) and PI-treated mice (hatched bars) mice were also treated daily with ADP355 (1 mg/kg) or PBS via intraperitoneal injection for the final 14 days of PI exposure, after which all mice were euthanatized and subcutaneous inguinal and visceral epididymal fat pads were collected and weighed. Data are means ± S.E.M. of fat pad mass in milligrams, and were generated from 9–21 mice per group. a Inguinal fat depot weight in vehicle and lopinavir/ritonavir-treated mice following administration of PBS or ADP355. *** and ** indicates significant (p<0.001 and p<0.01) decreases in weight of the inguinal fat depot in lopinavir/ritonavir/PBS and lopinavir/ritonavir/ADP355 mice as compared to vehicle-treated mice, respectively. # indicates significant (p<0.05) increase in weight of the epididymal fat depot in lopinavir/ ritonavir/ADP355 mice compared to lopinavir/ritonavir/PBS mice. b Epididymal fat depots in vehicle and lopinavir/ritonavir-treated mice following administration of PBS or ADP355. *** and ** indicates significant (p<0.001 and p<0.01, respectively) decreases in weight of the inguinal fat depot in lopinavir/ritonavir/ADP355 and lopinavir/ritonavir/PBS mice as compared to vehicle-treated mice, respectively
