Figure 2. Interactions of NAPE-PLD with NAPE and bile acids.
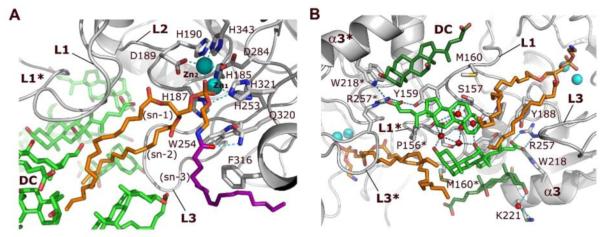
(A) Binding of NAPE to the active site of NAPE-PLD. A molecule of PE (carbon atoms in orange), which derives from the expression system used to produce the enzyme, occupies the binding site reserved to the PE fragment of NAPE. Its phosphate group bidentately bridges the two Zn2+ of the metal center (Zn1 and Zn2). Aspartate D284 bridges the two metal ions while three histidine residues (H185, H187 and H253) fill the coordination sphere of Zn1, and residues D189, H190, and H343 fill the coordination sphere of Zn2. A possible orientation of the N-acyl (oleoyl) substituent of NAPE is shown (carbons drawn in purple). (B) Specific binding sites for steroid acids. Two pairs of DC molecules (carbons colored in green) bind to the L1 loops at the interface of NAPE-PLD with the membrane. The central pair (carbons colored light green) forms a primary micelle of bile acids that exposes its hydrophobic convex side to the membrane interface. These steroid acids expand the hydrophobic surface of the opposite subunit via their steroid A-rings (Supplementary Fig. 1), and interact directly with the fatty acyl chains of PE. A network of H-bonds (dotted lines) for the bile acid carboxylate groups is arranged by the side chains of Y188, W218, and R257, together with that of Y159 of the opposite subunit. Two additional DC molecules (carbons colored dark green) form tight van der Waals contacts with the protein and expose their hydrophilic concave sides. This pairs contributes to orient the steroid carboxylates of the central DC pair (carbons colored light green). Red spheres symbolize water molecules involved in hydrogen bonding. Asterisks indicate residues and loops of the opposite dimer subunit.
