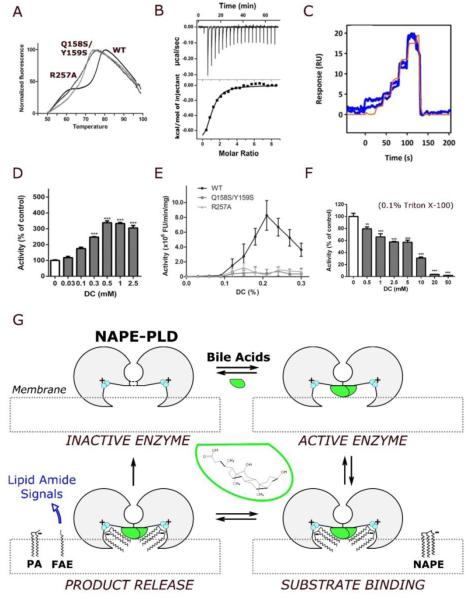
Figure 3. Bile acids regulate dimer formation and catalytic activity.
(A) Representative fluorescence thermal shift profiles for the stabilization of the NAPE-PLD dimer in the presence of DC (0.2% w/v). The analysis yields a melting temperature Tm = 77°C for the wild-type enzyme, and Tm = 71°C and 70°C for the mutants R257A and Q157S/Y158S, respectively (values are means for 5 replicates). (B) Binding thermodynamics profile for the titration of DC against NAPE-PLD obtained by isothermal titration calorimetry (ITC) at 25°C. Each peak corresponds to one injection of DC (top panel, raw titration data; bottom panel, integration of the data, corrected for heat of dilution). Microcalorimetric analysis yields a KD=38.5(±0.8) μM, ΔH=−1.2(±0.2) kcal/mol, and TΔS=−4.9(±0.3) kcal/mol (errors quoted standard deviations of n=3 experiments). (C) Profiles of the binding kinetics for DC on immobilized NAPE-PLD obtained at 25°C by surface plasmon resonance (SPR) with FastStep injections. Analysis (of n=3 replicates) yields a KD=44(2) μM, and dissociation constant Kd=0.44(3) s−1. (D) Concentration-dependent activation of NAPE-PLD by DC. The activity of purified human recombinant NAPE-PLD was measured in the absence of other detergents, using a liquid chromatography/mass spectrometry-based assay (LC/MS). (E) In the absence of synthetic detergent, DC stimulates the activity of wild-type (WT) human recombinant NAPE-PLD (0.1% w/v DC ≈ 2.4 mM DC), but not of NAPE-PLD mutants R257A and Q158S/Y159S. Enzyme activity was measured using a fluorescence-based assay (Methods). (F) In the presence of Triton X-100 (0.1%), DC inhibits the activity of wild-type enzyme using a liquid chromatography/mass spectrometry-based assay (Methods). (G) Hypothetical mechanism of NAPE-PLD activation. According to this model, illustrated clockwise from top left, bile acids (green) promote the assembly of inactive NAPE-PLD subunits (gray) into an active dimer. The resulting lipid-protein complex can recognize NAPE species at the membrane interface. The positively charged binuclear zinc center (cyan) coordinates anionic NAPEs, generated by N-acylation of zwitterionic PE, and orchestrates their hydrolysis to form bioactive FAEs along with phosphatidic acid (PA). A diversity of bioactive FAEs can be generated similarly that activate membrane GPCRs (e.g. cannabinoid receptors, CBs) or nuclear PPARs.