Table 3.
[CoII(Por)]-catalysed trans-selective β-lactam synthesis from αdiazocarbonyl compounds.[a]
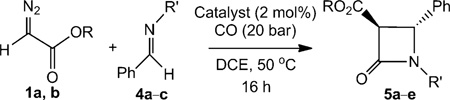 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Diazo | Imine | Yield [%][b] (trans:cis) |
| 1 | [CoII(P1)] | R = Et | R′ = Me | 65 (5a) (>95:5) |
| 2 | [CoII(P2)] | R = Et | R′ = Me | 65 (5a) (>95:5) |
| 3 | [CoII(P3)] | R = Et | R′ = Me | 67 (5a) (>95:5) |
| 4 | [CoII(P1)] | R = Et | R′ = CH2Ph | 50 (5b) (>90:5) |
| 5 | [CoII(P1)] | R = Et | R′ = tBu | 55 (5c) (>95:5) |
| 6 | [CoII(P1)] | R = tBu | R′ = Me | 66 (5d) (>95:5) |
Stoichiometry EDA:imine = 1:2.
Isolated yields after column chromatography.
