Abstract
This study examined whether neighborhood social environment was related to patterns of adherence to oral hypoglycemic agents among primary care patients with type 2 diabetes mellitus. Residents in neighborhoods with high social affluence, high residential stability and high neighborhood advantage compared to residents in neighborhoods with one or no high features present, were significantly more likely to have an adherent pattern compared to a nonadherent pattern. Neighborhood social environment may influence patterns of adherence. Reliance on a multi-level contextual framework, extending beyond the individual, to promote diabetic self-management activities may be essential for notable public health improvements.
Keywords: primary health care, type 2 diabetes, neighborhood social environment, medication adherence
Introduction
Despite the development of effective pharmacological therapy to prevent both macrovascular and microvascular complications and adverse events1-4 diabetes control remains sub-optimal.5-7 Poor adherence to recommended regimens is a factor in preventable morbidity and mortality in diabetic patients.8,9 While individual characteristics are important contributors to medication adherence, much of the observed variation in adherence rates remains unexplained by these factors.10-12 Neighborhood environment may shape medication adherence through many factors such as socioeconomic resources, perceptions, expectations and beliefs. Individuals living in the same neighborhood may be more similar to each other than persons living in other neighborhoods because they share common social, economic, systemic, and lifestyle characteristics. Thus there may be common health behaviors that persist over and above individual variation and relate to living environment.13,14 The assessment of this collective phenomenon is needed to fully elucidate and understand adherence behaviors. However, little empirical knowledge exists about the nature and size of these collective or contextual neighborhood level effects on health behaviors such as medication adherence.15
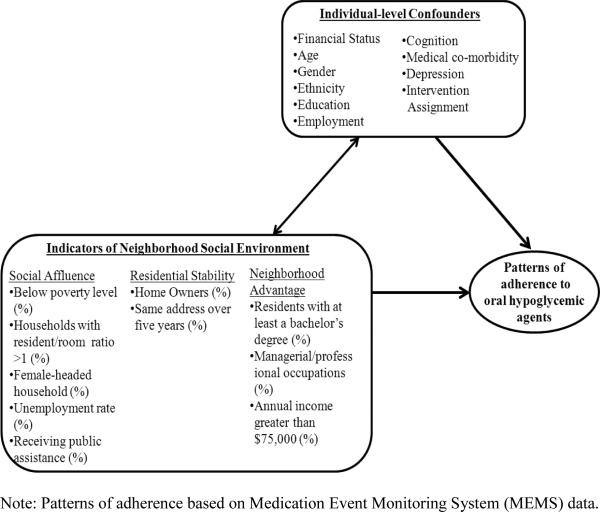
Neighborhood social environment is an important construct in discerning how neighborhood contextual effects influence health behavior.16 With a movement to examine neighborhood effects beyond the influence of poverty, a focus on social characteristics, organization and culture in relation to behaviors and outcomes has become essential.17 The concepts of social affluence, neighborhood advantage and residential stability, derived from the work of Sampson et al.,18-20 have been the subject of much research and have been established as important explanatory factors in understanding the role of neighborhood social environment in health.21-23 These measures tap into both the influence of poverty as well as social mechanisms and processes hypothesized to link neighborhood environment to health. We seek to understand whether these constructs are related to medication adherence, a critical predictor of prognostic outcomes particularly for patients with diabetes. Our conceptual framework, shown in Figure 1, depicts the key constructs assessed in this study relating key features of the neighborhood social environment to patterns of adherence over time.
Figure 1.
Proposed conceptual model of indicators of neighborhood social environment and patterns of adherence to oral hypoglycemic agents. This model was adapted from the work of the Translating Research Into Action for Diabetes (TRIAD) study 26 and Carpiano RM 52
Prior work has found that neighborhood residence is associated with medication adherence13,24,25 and other self-care behaviors,26-28 even when controlling for individual characteristics. However, these studies have been limited by their cross-sectional designs, reliance on subjective adherence assessments and/or lack of a representative sampling frame due to regional variations in culture, context and available resources. Few identified studies investigated this relationship among diabetic patients, a population for whom the environment may have a particularly salient role. Our work extends current findings by employing a longitudinal study design to examine adherence with medication regimens as assessed by an objective and time-varying measure of adherence among a diverse sample of primary care patients with diabetes. Demonstrating a relationship between features of neighborhood social environment and patterns of adherence to oral hypoglycemic agents will set the stage for interventions targeting resources for persons and neighborhoods most at risk for poor health.
Within a prospective randomized controlled trial we sought to investigate whether indicators of neighborhood social environment (social affluence, neighborhood advantage and residential stability) would be associated with patterns of adherence to oral hypoglycemic agents. Our aim was to examine whether residents in neighborhoods with high social affluence, high neighborhood advantage, and/or high residential stability would be more likely to have a pattern of adherence or increasing adherence over time. We hypothesized that residents in neighborhoods with two or three features present (high social affluence, high neighborhood advantage and high residential stability) would be more likely to have a pattern of adherence or a pattern of increasing adherence than residents in neighborhoods with one or none of these features present. To our knowledge, this is the first investigation to examine social affluence, neighborhood advantage and residential stability in relation to longitudinal patterns of adherence among primary care patients with diabetes.
Methods
Recruitment
The randomized controlled trial, A Brief Intervention to Improve Adherence through Integrated Management of Type 2 Diabetes Mellitus and Depression Treatment, was designed to assess whether an integrated care intervention in primary care improved glucose control and depressive symptoms in type 2 diabetes mellitus (type 2 DM).29 In all, 180 patients were recruited from three primary care practices in Philadelphia, Pennsylvania and were randomly assigned to the intervention or usual care. Patients with a diagnosis of type 2 DM and a prescription for an oral hypoglycemic agent within the past year were identified through electronic medical records from April 2010 to April 2011. Patients with an upcoming appointment were approached for additional screening. Eligibility criteria were: 1) aged 30 years and older; 2) a diagnosis of type 2 DM; and 3) a current prescription for an oral hypoglycemic agent. Exclusion criteria were: 1) inability to give informed consent; 2) significant cognitive impairment at baseline (Mini-Mental State Examination (MMSE) <21);30 3) residence in a care facility that provides medications on schedule; and 4) unwillingness or inability to use the Medication Event Monitoring System (MEMS). The study protocol was approved by the University of Pennsylvania, Perelman School of Medicine Institutional Review Board. The intervention is described in detail elsewhere.29
Study Design
The trial was conducted in two phases: the run-in phase and the randomized controlled trial phase. The first phase of this trial consisted of a 2-week run-in phase to obtain pre-intervention adherence data for all patients. Baseline demographics and glycated hemoglobin (HbA1c) assays to measure glycemic control were also collected at this time. Phase 2 of the study, where patients were randomized to the integrated care intervention or usual care, commenced after the 2-week run-in phase and occurred over 12 weeks.
Intervention
Integrated care managers worked with patients individually in the intervention group to offer education, guideline-based treatment recommendations, and monitor adherence and clinical status in collaboration with physicians. The integrated care manager addressed factors involved in adherence to oral hypoglycemic agents. The patient-level factors resulting in nonadherence included depression, chronic medical conditions, function, cognition, social support, cost of medications, side effects, and past experiences with medications. We chose this multi-faceted approach because education alone has not been found to be effective for improving adherence.31
The intervention was presented to patients as a supplement to, rather than a replacement for, existing primary care treatment. Over a three month period participants had three 30-minute in person sessions (baseline, 6 weeks and 12 weeks) and two 15-minute telephone monitoring contacts. Integrated care managers were two research coordinators (one Master's level and one bachelor's level) who administered all intervention activities. The integrated care managers received training on pharmacotherapy for type 2 DM management during weekly clinical sessions with the principal investigator prior to trial initiation.
Usual Care
Patients in the usual care group underwent the same assessments at the same time points (baseline, 6, and 12 weeks) as the patients in the integrated care intervention. Research assistants conducted all assessments in-person and were blinded to patients’ randomization status.
Measurement Strategy
Patient Characteristics
Potential study patients were screened for cognitive impairment using the MMSE, a short standardized mental status examination widely employed for clinical and research purposes.32 At baseline, sociodemographic characteristics were assessed using standard questions. Address data was obtained for participants at baseline. Electronic monitoring data obtained from the MEMS Caps were used to measure adherence to oral hypoglycemic agents. Adherence was assessed as the proportion of medication MEMS cap openings in a given week relative to the prescribed doses for the week. Blood glycemic control was assessed at baseline and 12 weeks in accordance with American Diabetes Association Guidelines.33 The in2it A1C Analyzer provides point of care testing and was used to obtain glycated hemoglobin (HbA1c) assays. This device has acceptable precision and agreement in comparison with laboratory services 34.
Neighborhood Social Environment
Individual patient residential data was geo-coded at the tract level and then was merged with 2010 tract-level Census data. Factor analysis was conducted on 13 variables as done in prior work by Sampson et al.18-20 and others to assess key constructs of the social environment: social affluence, neighborhood advantage and residential stability.12,18,21-23,35 Factor analysis examines the nature of the relationships between variables by identifying the smallest number of factors explaining composites of the observed variables. To decrease collinearity between resulting factors we required that variables loaded above 0.55 on a single factor. All 13 variables loaded above 0.55 on single factor resulting in three single composite factors/variables. Conventional diagnostics such as scree plots also confirmed these three identified factors. These factors represented constructs of neighborhood social environment: social affluence, neighborhood advantage, and residential stability. Social affluence was derived from five variables: percent of households with resident/room ratio greater than 1 (factor loading = 0.57), percent of female-headed households (0.84), percent unemployed (0.77), percent of people below the poverty line (0.87), and percent of people receiving public assistance (0.73). Neighborhood advantage was derived from three variables: percent of residents with at least a bachelor's degree (0.87), percent of people in professional occupations (0.75), and percent of people with a household income greater than $75,000 (0.67). Finally, residential stability was derived from two variables: the percent of house owners (0.86) and the percent of residents living at the same address over 5 years (0.86). Factor scores for neighborhood social environment (social affluence, neighborhood advantage and residential stability) were dichotomized as high or low based on the sample median.
Analysis
This analysis was conducted employing classifications of patients into patterns of adherence to oral hypoglycemic agents, as per our prior work.36 In brief, we employed general growth curve mixture models (GGCMM)37-43 to generate estimated posterior probabilities of unobserved class membership for each patient. This seminal approach improves precision by accounting for both intervention effects and baseline covariates on adherence over time. Longitudinal patterns of adherence to oral hypoglycemic agents were created by classifying patients based on the largest posterior probability of membership across the classes. The resulting categorical variable, patterns of adherence to oral hypoglycemic agents (adherent, increasing adherence, and nonadherent), was employed as the dependent variable for this analysis.
Multinomial logistic regression related neighborhood social environment (social affluence, neighborhood advantage and residential stability) to patterns of adherence to oral hypoglycemic agents. Results are presented in the form of odds ratios and 95% confidence intervals. Neighborhood social environment was assessed categorically. Consistent with prior work, the model included terms to adjust for age, ethnicity, gender, educational attainment, financial status, employment, frequency of medication administration, number of medical conditions, cognitive status, practice, baseline HbA1c, and intervention condition.44 We set α at 0.05, recognizing that tests of statistical significance are approximations that serve as aids to inference. The GGCMM was fitted using Mplus version 7 (Muthén & Muthén) 45 and other analyses were conducted in STATA version 12 for Windows (STATA Corporation, College Station, TX).
Results
Study sample
The CONSORT flow diagram for the Brief Intervention to Improve Adherence through Integrated Management of Type 2 Diabetes Mellitus and Depression Treatment trial has been published elsewhere.29 In brief, of 715 patients with type 2 DM identified by electronic medical records, 265 were eligible and were approached and 190 were enrolled (71.7% participation rate). Consent was followed by a 2-week run-in phase in which adherence to medications was assessed. At the 2-week visit, 5 physicians had discontinued the antidepressant, 1 physician discontinued the oral hypoglycemic agent, and 2 patients were lost to follow-up. In all, 182 patients were randomized to the integrated care intervention or usual care. After randomization at the 2-week meeting, 2 patients in the integrated care intervention were lost to follow-up leaving 180 patients who completed the final study visit for our study sample.
In all, 179 patients had complete data on residential address and covariates of interest and were included in the present analysis. The mean age of our sample was 57.4 years (standard deviation (s.d.) 9.5 years). One hundred and twenty-one (67.6%) of the patients were women. The self-identified ethnicity of patients was 65 white (36.3%), 101 African-American (56.4%), 7 Hispanic (3.9%), and 6 (3.4%) who self-identified as ‘other.’ In all, 69 patients (38.6%) were married, and 29 patients (16.2%) had less than a high school education. The mean number of medical conditions was 7.3 (s.d. 2.4) and the mean MMSE score was 28.2 (s.d. 2.3). Social affluence, residential stability, and neighborhood advantage stratified by median factor score as High and Low are depicted in Table 1.
Table 1.
Social affluence, residential stability, and neighborhood advantage stratified by median factor score (High versus Low).
| Neighborhood Indicators | High (n=89) | Low (n=90) |
|---|---|---|
| Social Affluence | ||
| Poverty (%), mean (s.d.) | 13.52 (1.39) | 35.60 (1.22) |
| Households with resident/room ratio > 1 (%), mean (s.d.) | 1.00 (.12) | 2.89(.29) |
| Female-headed households (%), mean (s.d.) | 9.87 (.69) | 29.71 (.75) |
| Unemployment rate (%), mean (s.d.) | 7.1 (.39) | 18.7 (.70) |
| Receiving public assistance (%), mean (s.d.) | 2.22 (.18) | 11.03 (.53) |
| Residential Stability | ||
| House owners (%), mean (s.d.) | 60.39 (2.94) | 51.77 (1.60) |
| Same address over 5 years (%), mean (s.d.) | 61.49 (1.06) | 57.96 (2.06) |
| Neighborhood Advantage | ||
| Residents with at least a bachelor's degree (%), mean (s.d.) | 43.31 (2.21) | 11.29 (.79) |
| Managerial/professional occupations (%), mean (s.d.) | 13.85 (.66) | 9.2 (.42) |
| Annual income greater than $75,000 (%), mean (s.d.) | 38.70 (1.95) | 12.24 (.81) |
Note: s.d=standard deviation. Data obtained from 2010 U.S. Census and all measures are defined in accordance with these guidelines.
Neighborhood social environment (residential stability, social affluence, and neighborhood advantage) and patterns of adherence to oral hypoglycemic agents
We examined the relationship between composite neighborhood characteristics and patterns of adherence to oral hypoglycemic agents (Table 2). The column of Table 2 labeled Adherent vs. Nonadherent provides odds ratios estimating the association of neighborhood social environment with patterns of adherence, comparing the adherent pattern to the nonadherent pattern. Compared to residents in neighborhoods with one or no high features present, residents in neighborhoods with high social affluence, high residential stability, and high neighborhood advantage were more likely to have an adherent pattern compared to a nonadherent pattern (adjusted odd ratio (OR)= 8.48, 95% confidence interval (CI) [1.71, 42.02]). The column of Table 2 labeled Increasing Adherence vs. Nonadherent compares the increasing adherence pattern to the nonadherent pattern. Compared to residents in neighborhoods with one or no high features present, residents in neighborhoods with high social affluence, high residential stability, and high neighborhood advantage were more likely to have an increasing adherence pattern compared to a nonadherent pattern (adjusted OR= 12.91, 95% CI [2.20, 75.80]). There was not a significant relationship between neighborhoods with two features present and patterns of adherence.
Table 2.
Multinomial logistic regression of neighborhood characteristics and three patterns of adherence (n= 179).
| Adherent vs. Nonadherent OR [95% CI] | Increasing Adherence vs. Non adherence OR [95% CI] | |
|---|---|---|
| Neighborhood characteristics | ||
| High social affluence, high residential stability, and high neighborhood advantage (n=41) | 8.48* [1.71, 42.02] | 12.91* [2.20, 75.80] |
| High social affluence and high neighborhood advantage (n=36) | 2.44 [0.67, 8.86] | 1.61 [0.35, 7.54] |
| High residential stability and high neighborhood advantage (n=10) | 4.05 [0.28, 57.60] | 1.86 [0.12, 28.54] |
| High social affluence and high residential stability (n=12) | 1.78 [0.29, 10.75] | 0.50 [0.05, 5.56] |
| One or fewer high features present: stability, affluence, or advantage (n=80) | 1.00 | 1.00 |
Note: OR = odds ratio; CI = confidence interval
p< .05
All estimates are adjusted for age, ethnicity, gender, educational attainment, financial status, employment, frequency of medication administration, number of medical conditions, cognitive status, practice, baseline glycated hemoglobin (HbA1c), depression, and intervention condition.
Discussion
The principal finding of this study is that residents in neighborhoods with high social affluence, high residential stability, and high neighborhood advantage were much more likely to have an adherent pattern compared to a nonadherent pattern. Similarly, residents in neighborhoods with high social affluence, high residential stability, and high neighborhood advantage were much more likely to have an increasing adherence pattern compared to a nonadherent pattern. These results provide evidence that features of neighborhood social environment may be important contributors to patterns of adherence to oral hypoglycemic agents, a critical factor in treatment effectiveness and subsequent outcomes.
Before we discuss our findings, the results must be considered in the context of several potential study limitations. First, data was collected from three primary care sites whose patients may not be representative of other primary care practice settings. However, they were similar to other primary care practices in the region in terms of diversity and size. Second, it is important to note that all methods for assessing adherence have limitations. We utilized MEMS caps as our primary measure because they are an objective measure, have a low failure rate,46 and are more sensitive than other measures.47 Both groups (intervention and usual care) would experience any influence of MEMS caps on medication adherence equally. Third, we solely examined the role of neighborhood social environment on behavioral patterns (medication adherence). Future research could incorporate other measures of neighborhood environment (e.g. physical environment: built environment, local food environment) as well as individual factors (physical health, psychosocial stress, and psychosocial resources) and health outcomes in order to delve deeper into the complex interplay of mediating or moderating pathways linking neighborhoods to health across time. Finally, we are constrained by the utilization of an administrative definition of neighborhoods (census tracts), which may not be the most meaningful level of aggregation. It is possible that assessment within a more respondent-derived neighborhood context may elicit the greatest explanatory power in understanding the role of neighborhood environment.48
Despite these limitations, our results are important to consider given that this study is one of the first examine the relationship between neighborhood social environment, as assessed by social affluence, residential stability, and neighborhood advantage, and patterns of adherence. A growing body of evidence has linked indicators of neighborhood social environment, namely measures of socioeconomic status with morbidity and mortality.49 However, little research has examined more proximal mechanisms to health, health behaviors, which are critical precedents for understanding disease prognosis in diabetes. In our work, we characterize mechanisms shaping the link between neighborhood social environment and health21,22 demonstrating that the presence of multiple features (high social affluence, high residential stability, and high neighborhood advantage) may be critical in understanding adherence patterns. Our results support evidence that neighborhoods matter and furthermore help to inform and enhance future research on how neighborhoods matter.
While we found that residents in neighborhoods with three high features present were significantly more likely to have a pattern of adherence or a pattern of increasing adherence, we did not find that residents in neighborhoods with two features present were significantly more likely to have a pattern of adherence or a pattern of increasing adherence. This aligns with work demonstrating that accumulation of exposures to multiple contextual factors may explain the health impact of neighborhoods. While the presence of a few features is somewhat influential, the presence of multiple features for extended periods of time is associated with the greatest health impact.50 Because fewer neighborhood features may have only a modest effect, our study may not have been large enough to provide an adequate test of our hypothesis that residents in neighborhoods with two features present were significantly more likely to have a pattern of adherence or a pattern of increasing adherence than residents in neighborhoods with one or no features present.
Our findings are consistent with the work of Billmek et al. in which nonadherence to medications was examined in relation to neighborhood deprivation among persons with type 2 DM. Billmek and colleagues examined the cross-sectional relationship of neighborhood deprivation, as assessed by the Neighborhood Socioeconomic Status Index comprised of census tract measures, and self-reported adherence. Their findings suggest that social environment as well as related costs may contribute to nonadherence.24 In our work we enrolled patients who already had filled prescriptions for oral hypoglycemic agents and we adjusted for individual financial status, thus minimizing the influence of cost on adherence to medication regimens, and supporting evidence that neighborhood social environment and adherence may be linked by factors other than financial pressure. Our work is further delineated from prior work by a focus on features of neighborhood social environment derived from the work of Sampson et al.18-20 Our findings provide insight into the social mechanisms and process that link neighborhood environment to health. Furthermore, we used an objective measure of adherence, and our use of general growth curve mixture models allowed us to distinguish distinct patterns of adherence over time instead of assessing adherence through proportions at singular point(s) in time with no assessment of variation over time and group classification. Our findings extend prior work by demonstrating that features of the social environment are associated with longitudinal patterns of adherence as assessed by objective measures of medication adherence among primary care patients with type 2 DM.
Our results highlight the significance of features of the social environment in shaping patterns of adherence to oral hypoglycemic agents over time, over and above of the effects of individual characteristics. A framework through which we can characterize the underlying mechanisms relating neighborhood social environment to adherence has been established.51,52 Following this framework, a lack of social capital and/or cohesion may exist in neighborhoods with low social affluence, advantage, and stability.53 Such environments may lack a myriad of health promoting social processes such as social control over deviant health-related behavior and attitudes (e.g. over-eating). These environments may promote unhealthy behaviors through social norms,54 minimal levels of social trust 55 and a lack of a supportive community environment.16 Furthermore, the character of daily routines, shaped by neighborhood environment (e.g. disorder), dictates the availability of temporal windows to engage in health promoting activities such as medication taking.56 Such windows may be limited in more compromised neighborhoods. All these processes may be at work in shaping patterns of health behavior over time, particularly when multiple features are present cumulatively over the life course.57,58
This study is among the first to suggest that features of neighborhood social environment may influence medication adherence. While the distinct mechanisms for this association require further examination, this study adds to a growing body of evidence that patient's social environment influences behavioral patterns. While such contextual factors play a critical role in shaping health outcomes, they are seldom addressed or incorporated into treatment plans. As a result, patients who receive guideline concordant care may not achieve treatment targets due to factors related to their daily environmental context. Reliance on a multi-level contextual framework, extending beyond the individual, to promote diabetic self-management activities may be essential for effective intervention deployment and notable public health improvements.59-61 Ongoing efforts to improve access and quality of care should be accompanied by initiatives to integrate the health care systems within community settings. Collaborative networks between healthcare systems and neighborhood communities are needed to foster effective adherence initiatives.
Acknowledgements
None
Research Support
This work was supported by American Heart Association Award #13GRNT17000021, National Institute of Mental Health R21 MH094940, and a National Institute of Mental Health R34 MH085880. Dr. Small was supported by grant SES-1260782 from the Measurement, Methodology and Statistics Program of the U.S. National Science Foundation.
Footnotes
Trial Registration: clinicaltrials.gov registration number: NCT01098253
References
- 1.UK Prospective Diabetes Study Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998 Sep 12;352(9131):854–865. [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000 Aug 12;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 5.Saaddine JB, Cadwell B, Gregg EW, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988-2002. Ann Intern Med. 2006 Apr 4;144(7):465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Egede LE, Gebregziabher M, Hunt KJ, et al. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care. 2011 Apr;34(4):938–943. doi: 10.2337/dc10-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services Healthy People 2020. doi: 10.3109/15360288.2015.1037530. http://www.healthypeople.gov. [DOI] [PubMed]
- 8.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Jama. 2007 Jan 10;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 10.Geraghty EM, Balsbaugh T, Nuovo J, Tandon S. Using Geographic Information Systems (GIS) to assess outcome disparities in patients with type 2 diabetes and hyperlipidemia. Journal of the American Board of Family Medicine : JABFM. 2010 Jan-Feb;23(1):88–96. doi: 10.3122/jabfm.2010.01.090149. [DOI] [PubMed] [Google Scholar]
- 11.Laraia BA, Karter AJ, Warton EM, Schillinger D, Moffet HH, Adler N. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE). Soc Sci Med. 2012 Apr;74(7):1082–1090. doi: 10.1016/j.socscimed.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long JA, Field S, Armstrong K, Chang VW, Metlay JP. Social capital and glucose control. J Community Health. 2010 Oct;35(5):519–526. doi: 10.1007/s10900-010-9222-0. [DOI] [PubMed] [Google Scholar]
- 13.Groenewegen PP, Leufkens HG, Spreeuwenberg P, Worm W. Neighbourhood characteristics and use of benzodiazepines in The Netherlands. Soc Sci Med. 1999 Jun;48(12):1701–1711. doi: 10.1016/s0277-9536(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 14.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21:171–192. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 15.Berkman LF, Kawachi I. Social Epidemiology. Oxford University Press; New York, NY: 2000. [Google Scholar]
- 16.Kawachi I, Berkman LF. Social cohesion, capital, and health. In: Berkman LF, Kawachi I, editors. Social Epidemiology. Oxford University Press; New York, NY: 2000. [Google Scholar]
- 17.Browning CR, Cagney KA. Moving beyond poverty: neighborhood structure, social processes, and health. J Health Soc Behav. 2003 Dec;44(4):552–571. [PubMed] [Google Scholar]
- 18.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997 Aug 15;277(5328):918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 19.Sampson RJ, Morenoff JD, Earls F. Beyond social capital: spatial dynamics of collective efficacy for children. Am Sociol Rev. 1999;64(5):633–660. [Google Scholar]
- 20.Sampson RJ, Raudenbush SW. Systemic social observation of pubilc spaces: a new look at disorder in urban neighborhoods. American Journal of Sociology. 1999;105:603–652. [Google Scholar]
- 21.Yang TC, Matthews SA, Shoff C. Individual health care system distrust and neighborhood social environment: how are they jointly associated with self-rated health? J Urban Health. 2011 Oct;88(5):945–958. doi: 10.1007/s11524-011-9561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews SA, Yang TC. Exploring the role of the built and social neighborhood environment in moderating stress and health. Ann Behav Med. 2010 May;39(2):170–183. doi: 10.1007/s12160-010-9175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boardman JD. Stress and physical health: the role of neighborhoods as mediating and moderating mechanisms. Soc Sci Med. 2004 Jun;58(12):2473–2483. doi: 10.1016/j.socscimed.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Billimek J, August KJ. Costs and Beliefs: Understanding Individual- and Neighborhood-Level Correlates of Medication Nonadherence Among Mexican Americans With Type 2 Diabetes. Health Psychol. 2013 Dec 2; doi: 10.1037/hea0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merlo J, Lynch JW, Yang M, et al. Effect of neighborhood social participation on individual use of hormone replacement therapy and antihypertensive medication: a multilevel analysis. Am J Epidemiol. 2003 May 1;157(9):774–783. doi: 10.1093/aje/kwg053. [DOI] [PubMed] [Google Scholar]
- 26.Gary TL, Safford MM, Gerzoff RB, et al. Perception of neighborhood problems, health behaviors, and diabetes outcomes among adults with diabetes in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2008 Feb;31(2):273–278. doi: 10.2337/dc07-1111. [DOI] [PubMed] [Google Scholar]
- 27.Deshpande AD, Baker EA, Lovegreen SL, Brownson RC. Environmental correlates of physical activity among individuals with diabetes in the rural midwest. Diabetes Care. 2005 May;28(5):1012–1018. doi: 10.2337/diacare.28.5.1012. [DOI] [PubMed] [Google Scholar]
- 28.Adams MA, Ding D, Sallis JF, et al. Patterns of neighborhood environment attributes related to physical activity across 11 countries: a latent class analysis. The international journal of behavioral nutrition and physical activity. 2013;10:34. doi: 10.1186/1479-5868-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med. 2012 Jan-Feb;10(1):15–22. doi: 10.1370/afm.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 31.Mundt JC, Clarke GN, Burroughs D, Brenneman DO, Griest JH. Effectiveness of antidepressant pharmacotherapy: the impact of medication compliance and patient education. Depress Anxiety. 2001;13(1):1–10. doi: 10.1002/1520-6394(2001)13:1<1::aid-da1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association Clinical Practice Recommendations. Diabetes Care. 2014;37(Supplement 1) [PubMed] [Google Scholar]
- 34.Moridani MY, Verjee Z, Allen LC. Analytical evaluation of hemoglobin A(1c) dual kit assay on Bio-Rad Variant II: an automated HPLC hemoglobin analyzer for the management of diabetic patients. Clin Biochem. 2003;36(4):317–320. doi: 10.1016/s0009-9120(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 35.Brusilovskiy E, Salzer MS. A study of environmental influences on the well-being of individuals with psychiatric disabilities in Philadelphia, PA. Soc Sci Med. 2012 May;74(10):1591–1601. doi: 10.1016/j.socscimed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 36.de Vries McClintock HF, Morales KH, Small DS, Bogner HR. Patterns of adherence to oral hypoglycemic agents and glucose control among patients with type 2 diabetes mellitus. Behavioral Medicine. doi: 10.1080/08964289.2014.904767. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthen BO, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 38.Muthen B, Brown CH, Masyn K, et al. General growth mixture modeling for randomized preventive interventions. Biostatistics. 2002 Dec;3(4):459–475. doi: 10.1093/biostatistics/3.4.459. [DOI] [PubMed] [Google Scholar]
- 39.Muthen BO. Latent variable mixture modeling. In: Marcoulides GA, Schumacker RE, editors. New Developments and Techniques in Structural Equation Modeling. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2001. pp. 1–33. [Google Scholar]
- 40.Jo B, Muthen BO. Modeling of intervention effects with noncompliance: A latent variable approach for randomized trials. In: Marcoulides GA, Schumacker RE, editors. New Developments and Techniques in Structural Equation Modeling. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2001. pp. 57–87. [Google Scholar]
- 41.Elliott MR, Gallo JJ, Ten Have TR, Bogner HR, Katz IR. Using a Bayesian latent growth curve model to identify trajectories of positive affect and negative events following myocardial infarction. Biostatistics. 2005 Jan;6(1):119–143. doi: 10.1093/biostatistics/kxh022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ten Have TR, Elliott M, Joffe M, Zanutto E, Datto C. Causal models for randomized physician encouragement trials in treating primary care depression. Journal of the American Statistical Association. 2004;99:8–16. [Google Scholar]
- 43.Lin JY, Ten Have TR, Bogner HR, Elliott MR. Baseline patient characteristics and mortality associated with longitudinal intervention compliance. Stat Med. 2007 Dec 10;26(28):5100–5115. doi: 10.1002/sim.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogner HR, de Vries HF, O'Donnell AJ, Morales KH. Measuring concurrent oral hypoglycemic and antidepressant adherence and clinical outcomes. Am J Manag Care. 2013 Mar;19(3):e85–92. [PMC free article] [PubMed] [Google Scholar]
- 45.Muthén LK, Muthén BO. 2 ed. Muthén & Muthén; Los Angeles, CA: 1998. Mplus users guide. February 2001. [Google Scholar]
- 46.George CF, Peveler RC, Heiliger S, Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol. 2000;50:166–171. doi: 10.1046/j.1365-2125.2000.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074–1090. doi: 10.1016/S0149-2918(99)80026-5. discussion 1073. [DOI] [PubMed] [Google Scholar]
- 48.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001 Nov;91(11):1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001 Feb;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honold J, Beyer R, Lakes T, van der Meera E. Multiple environmental burdens and neighborhood-related health of city residents. Journal of Environmental Psychology. 2012;32(4):305–317. [Google Scholar]
- 51.Carpiano RM. Toward a neighborhood resource-based theory of social capital for health: can Bourdieu and sociology help? Soc Sci Med. 2006 Jan;62(1):165–175. doi: 10.1016/j.socscimed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 52.Carpiano RM. Neighborhood social capital and adult health: an empirical test of a Bourdieu-based model. Health Place. 2007 Sep;13(3):639–655. doi: 10.1016/j.healthplace.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Jencks C, Mayer SE. The social consequences of growing up in a poor neighborhood. In: Lynn LE, McGeary MGH, editors. Inner city poverty in the United States. National Academy Press; Washington, DC: 1990. [Google Scholar]
- 54.Macintyre S, MacIver S, Sooman A. Area, class, and health: Should we be focusing on places or people? Journal of Social Policy. 1993;22:213–234. [Google Scholar]
- 55.Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. J Health Soc Behav. 2001 Sep;42(3):258–276. [PubMed] [Google Scholar]
- 56.Takahashi LM, Wiebe D, Rodriguez R. Navigating the time-space context of HIV and AIDS: daily routines and access to care. Soc Sci Med. 2001 Oct;53(7):845–863. doi: 10.1016/s0277-9536(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 57.Dannefer D. Cumulative advantage/disadvantage and the life course: cross-fertilizing age and social science theory. J Gerontol B Psychol Sci Soc Sci. 2003 Nov;58(6):S327–337. doi: 10.1093/geronb/58.6.s327. [DOI] [PubMed] [Google Scholar]
- 58.Ferraro KF, Shippee TP. Aging and cumulative inequality: how does inequality get under the skin? Gerontologist. 2009 Jun;49(3):333–343. doi: 10.1093/geront/gnp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glasgow RE, Strycker LA, Toobert DJ, Eakin E. A social-ecologic approach to assessing support for disease self-management: the Chronic Illness Resources Survey. J Behav Med. 2000 Dec;23(6):559–583. doi: 10.1023/a:1005507603901. [DOI] [PubMed] [Google Scholar]
- 60.Glasgow RE. A practical model of diabetes management and education. Diabetes Care. 1995 Jan;18(1):117–126. doi: 10.2337/diacare.18.1.117. [DOI] [PubMed] [Google Scholar]
- 61.Jack L, Jr., Liburd L, Spencer T, Airhihenbuwa CO. Understanding the environmental issues in diabetes self-management education research: a reexamination of 8 studies in community-based settings. Ann Intern Med. 2004 Jun 1;140(11):964–971. doi: 10.7326/0003-4819-140-11-200406010-00038. [DOI] [PubMed] [Google Scholar]