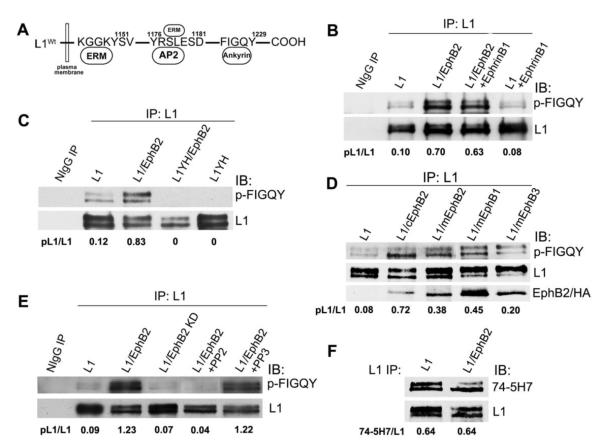
Figure 1. EphB2 receptor mediates tyrosine phosphorylation of L1 at FIGQY motif.
A. Location of tyrosine-containing motifs in the L1 cytoplasmic domain that bind ezrin-radixin-moesin (ERM) proteins, the clathrin adaptor AP2, and ankyrin. B-E. Immunoblotting (IB) with p-FIGQY antibody in L1 immunoprecipitates (IP) from transfected HEK293 cells or control precipitates with nonimmune IgG (NIgG). Ratios of phosphorylated L1 to L1 protein (pL1/L1) were obtained by densitometry after reprobing with L1 antibody.
(B) Expression of L1 and EphB2 increased L1 phosphorylation at FIGQY in transfected HEK293 cells compared to cells expressing only L1. Addition of ephrinB1 to cells did not further increase p-FIGQY in L1.
(C) p-FIGQY antibody did not recognize L1 immunoprecipitated from HEK293 cells transfected with L1Y1229H (L1YH) with or without EphB2 co-expression.
(D) Mouse mEphB2, mEphB1 (HA-tagged) and mEphB3 (HA-tagged) induced phosphorylation of L1 at FIGQY when expressed in HEK293 cells, while chick cEphB2 was the most effective (top panel). mEphB2, cEphB2, mEphB1-HA, and mEphB3-HA each co-immunoprecipitated with L1 as shown by immmunoblotting of L1 immunoprecipitates with EphB2 antibodies or anti-HA antibody (lowest panel).
(E) EphB2 kinase activity was required for phosphorylation of FIGQY, as EphB2 kinase dead mutant (EphB2 KD) did not induce p-FIGQY in L1-expressing HEK293 cells. The Src kinase inhibitor PP2 decreased phosphorylation of L1 in L1/EphB2 co-transfected HEK293 cells, while the nonfunctional analog PP3 had no effect.
(F) EphB2 did not induce tyrosine phosphorylation of L1 at Y1176RSL in HEK293 cells, as indicated by equal levels of non-phosphorylated Y1176RSL in L1 detected by 74-5H7 antibody, which specifically recognizes the non-phosphorylated motif.