Fig 1. Physico-chemical properties of chitosan hydrogel.
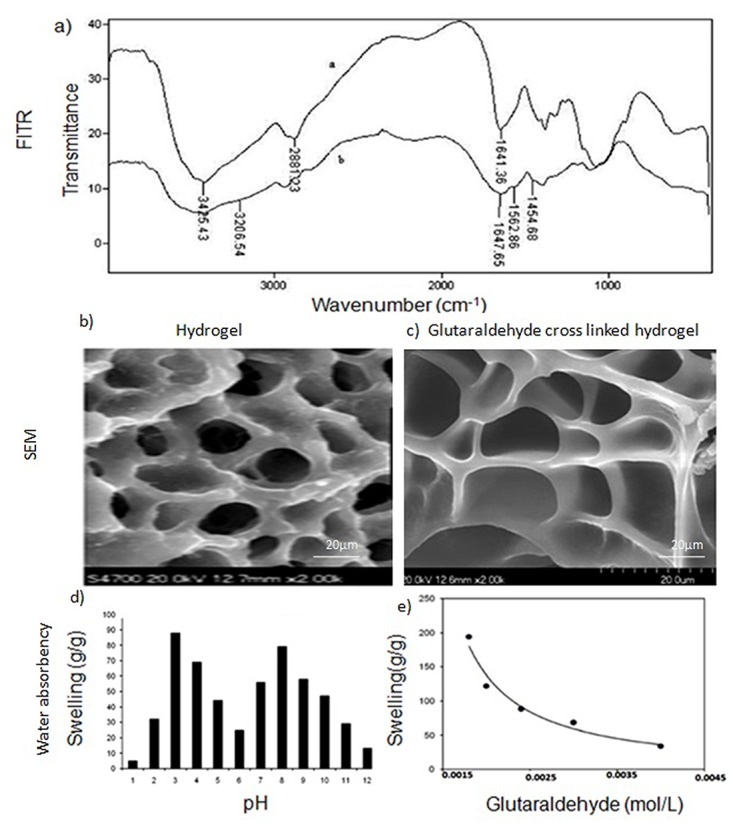
a) FTIR spectra of chitosan-based semi-IPN hydrogel. The broad band at 3425 cm-1 on the upper spectra corresponds to the associated-OH stretching vibrations of the hydroxyl groups. The peak at 1641 cm-1 corresponds to N-H deformation bending of chitosan. In the lower spectrum of the hydrogel, new peaks appeared at 3206, 1647, and 1562 cm-1 and these may be due to amide NH stretching, asymmetric, and symmetric amide NH bending, respectively. b&c) SEM images of the hydrogel and the glutaraldehyde cross linked chitosan-based hydrogel, respectively. Pore size of 110μm and loose architecture was observed for the latter. d) Effect of pH and cross linker concentration on water absorbency of the semi-IPN. The two sharp swelling capacity changes can be attributed to high repulsion of the-NH3+groups in acidic media and the-COO-groups in the basic media. However, at very acidic conditions (pH < 2), a screening effect of the counter ions (i.e., Cl-) shields the charge of the ammonium cations and prevents efficient repulsion. As a result, a remarkable decrease in equilibrium swelling was observed (gel collapsing). Around pH 5, the carboxylic acid component contributes as well. e) As the concentration of glutaraldelyde increases, the water absorbency of the super absorbent composite decreased. This is due to the decrease in space between the copolymer chains as the cross linker concentration increased.
