Figure 1. Design and Western blot analysis of secreted multimerized sFasL chimeric molecules.
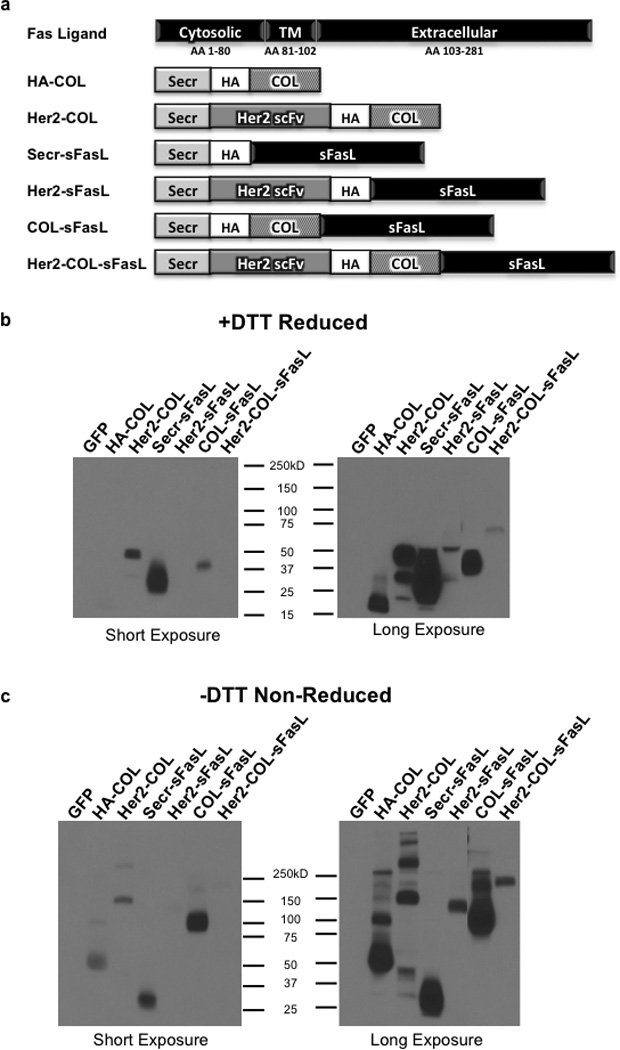
(a) Schematic representation of the sFasL chimeric molecules used in this study. A portion of the FasL extracellular domain (amino acids 139–281) was used to make constructs. Secr, optimal secretion signal; HA, hemagglutinin tag; COL, collagen-like trimerization domain; Her2 scFv, FRP5 Her2 single chain variable fragment; TM, transmembrane domain; AA, amino acids. (b and c) Western blot detection of chimeric sFasL molecules and control constructs under reducing and non-reducing conditions. HCT116 cells were transfected with mammalian expression constructs encoding the respective chimeric molecules. After 48 hours cell debris was removed from media and equal amounts of media subjected to Western blot analysis (b) with 25mM DTT and (c) without DTT. HA tag antibody was used for detection and data shown at two different exposure times for clarity. Western blot results are representative of three independent experiments.
