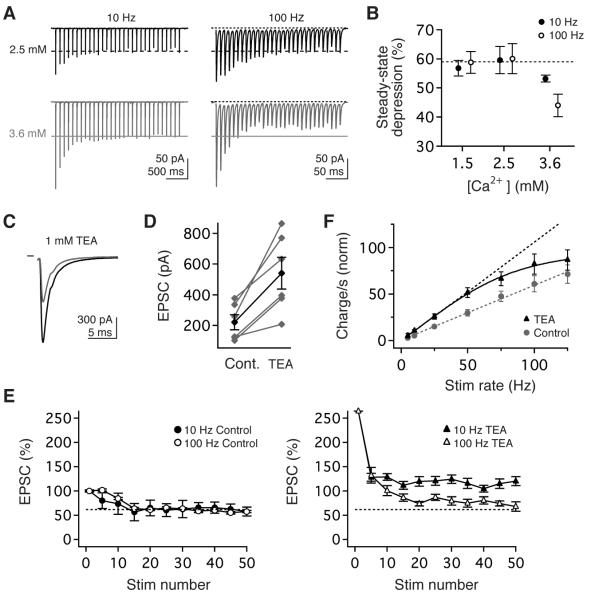
Figure 4.
Synaptic linearity requires low release probability and rapid repolarization of presynaptic action potentials. (A) Example EPSCs evoked in 2.5mM (top, black) and 3.6 mM (bottom, grey) Ca2+ by stimulus trains at 10 Hz (left) and 100 Hz (right). (B) The magnitude of steady-state transmission did not depend on stimulus rate or Ca2+ in 1.5 or 2.5 mM Ca, but depression was rate-dependent in 3.6 mM Ca2+. (C, D) Pharmacological blockade of fast presynaptic K currents with 1 mM TEA markedly increased peak EPSC amplitude. (E, left) Prior to TEA application, the magnitude of short-term depression was comparable for EPSCs evoked by10 Hz or 100 Hz stimuli. (E) Blockade of Kv3 and BK currents enhanced the magnitude of steady-state transmission and compromised rate-independence. (F) Normalized steady-state charge transfer per second before (circles, grey) and during TEA application (triangles, black), normalized to control EPSC1. Dashed lines are the linear extrapolations of data ≤ 50 Hz.