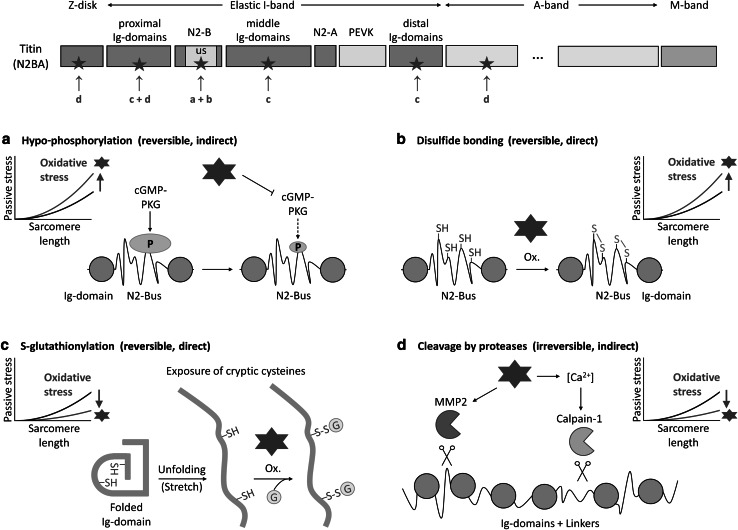
Fig. 2.
Oxidative stress-related modifications of titin affecting titin-based passive stiffness. The top panel illustrates the different segments of the titin chain (N2BA isoform) in a half-sarcomere, focusing on the various regions making up the elastic I-band segment. Segments where oxidative modifications occur are marked by arrows; the letters correspond to the respective type of oxidative modification indicated in panels (a–d). a Oxidative stress induces hypo-phosphorylation of the titin N2-Bus as it impairs NO-cGMP-PKG signalling; this modification increases titin stiffness. b Oxidizing conditions promote the formation of disulfide bonds in the titin N2-Bus; this modification increases titin stiffness. c Under oxidative conditions, buried cysteines in titin immunoglobulin (Ig-)domains are S-glutathionylated after they become exposed by domain unfolding (triggered by sarcomere stretch); this modification prevents domain refolding and thus reduces titin stiffness. d Oxidative stress increases the activity of proteases such as matrix metalloproteinase-2 (MMP2) and (via a rise in intracellular Ca2+ concentration) calpain-1, which degrade titin; these alterations would decrease titin stiffness