Abstract
Fibroepithelial lesions with cellular stroma are frequently termed cellular fibroadenomas although criteria for distinguishing them from a phyllodes tumor are vague and subjective. However, the clinical implications and surgical management for these 2 lesions may be different. We randomly selected 21 cases of fibroepithelial lesions sent in consultation to the senior author that were challenging to classify as cellular fibroadenoma or phyllodes tumor. One to 2 representative slides of each case along with patient age were sent to 10 pathologists who specialize in breast pathology. The World Health Organization criteria for phyllodes tumors and a diagnosis form were included with the study set. For the purposes of data reporting, fibroadenoma and cellular fibroadenoma are considered together. In only 2 cases was there uniform agreement as to whether the tumor represented a fibroadenoma or phyllodes tumor. Of the remaining 19 cases, if the diagnoses of fibroadenoma and benign phyllodes tumor were combined and separated from borderline and malignant phyllodes tumors, there was 100% agreement in 53% of cases and 90% agreement in 79% of cases. This study highlights the difficulty that exists in distinguishing some cellular fibroadenomas from phyllodes tumors even for pathologists who specialize in breast pathology. However, there appears to be considerable agreement when cellular fibroadenomas and benign phyllodes tumors are distinguished from borderline and malignant phyllodes tumors. Further studies are needed to determine if there is a clinically significant difference between cellular fibroadenomas and benign phyllodes tumors and how to better distinguish them from borderline and malignant phyllodes tumors.
Keywords: fibroepithelial lesion, phyllodes tumor, cellular fibroadenoma
Introduction
Fibroepithelial lesions of the breast with cellular stroma are frequently called cellular fibroadenomas and occasional cases can be difficult to distinguish from phyllodes tumors. While criteria have been established for diagnosing phyllodes tumors,1 many of these lesions have overlapping features and there is no single criterion to distinguish a fibroadenoma from a cellular fibroadenoma or a cellular fibroadenoma from a benign phyllodes tumor. This is not a trivial distinction as the clinical implications and surgical management for these lesions may be different at some centers. In this study, we attempted to determine the degree of inter-observer variability between pathologists in classifying a group of fibroepithelial lesions exhibiting varying degrees of stromal cellularity, atypia, mitotic activity, and intracanalicular growth.
Materials and Methods
The senior author (CR) searched the consultation files at Mayo Clinic spanning a 25-year period (1986-2010) for cases that were submitted for second opinion because of difficulty in classifying the lesion in question as a fibroadenoma or a phyllodes tumor. From this search, 21 of the identified cases were randomly selected for this study. One to 2 representative slides of each case along with patient age were sent to 10 pathologists who specialize in breast pathology. The 2003 World Health Organization (WHO) criteria for phyllodes tumor2 were included with the study set as well as a diagnosis form that included an “other” category (Table 1). All pathologists reviewed the same slides; no recuts were performed. Following review, each pathologist mailed the study set to the next pathologist on the list and diagnoses forms were returned independently to the first author only. For the purposes of data reporting, fibroadenoma and cellular fibroadenoma are considered together.
Table 1.
Distribution of the 10 Study Pathologists' Diagnoses for Each of the 21 Study Cases.
| Case No./Age (Years) | Fibroadenoma, n | Benign PT, n | Borderline PT, n | Malignant PT, n | Other |
|---|---|---|---|---|---|
| 1/21 | 5 | 5 | 0 | 0 | |
| 2/22 | 4 | 1 | 4 | 0 | Nodular PASH |
| 3/44 | 7 | 3 | 0 | 0 | |
| 4/65 | 0 | 1 | 7 | 2 | |
| 5/13 | 6 | 3 | 1 | 0 | |
| 6/40 | 6 | 3 | 0 | 0 | Fibroadenoma with phyllodal features |
| 7/22 | 8a | 1 | 0 | 0 | Hamartoma |
| 8/24 | 6 | 4 | 0 | 0 | |
| 9/54 | 1 | 6 | 3 | 0 | |
| 10/53 | 4 | 6 | 0 | 0 | |
| 11/39 | 6 | 2 | 1 | 0 | Sclerosing lobular hyperplasia |
| 12/38 | 3 | 3 | 3 | 0 | Periductal stromal sarcoma |
| 13/32 | 6 | 4 | 0 | 0 | |
| 14/53 | 8a | 2 | 0 | 0 | |
| 15/26 | 1 | 8 | 1 | 0 | |
| 16/36 | 10 | 0 | 0 | 0 | |
| 17/18 | 8 | 2 | 0 | 0 | |
| 18/32 | 6 | 2 | 2 | 0 | |
| 19/14 | 9 | 0 | 1 | 0 | |
| 20/16 | 7 | 3 | 0 | 0 | |
| 21/20 | 6 | 3 | 1 | 0 |
Abbreviations: PT, phyllodes tumor; PASH, pseudoangiomatous stromal hyperplasia.
In cases 7 and 14 several pathologists modified the fibroadenoma diagnosis to complex fibroadenoma.
Results
The pathologists’ diagnoses for each of the 21 study cases are listed in Table 1. In only 2 cases was there uniform agreement as to whether the tumor represented a fibroadenoma (case 16, Figure 1) or phyllodes tumor (case 4, Figure 2). However, in the phyllodes tumor case, subclassification varied from benign to malignant. Of the remaining 19 cases, in 4 cases the diagnoses were split nearly equally (5/5 or 6/4) between cellular fibroadenoma and benign phyllodes tumor (cases 1 and 10, Figures 3 and 4, respectively). Of note, in case 10 in which 6 pathologists diagnosed benign phyllodes tumor, of the 4 other pathologists, 2 diagnosed cellular fibroadenoma and 2 simply fibroadenoma. In 9 cases, the diagnoses ranged from fibroadenoma to borderline phyllodes tumor. In 4 of these cases, more than 1 pathologist made the diagnosis of borderline phyllodes tumor (cases 2 and 9, Figures 5 and 6, respectively). If the diagnoses of fibroadenoma/cellular fibroadenoma and benign phyllodes tumor were combined and separated from the borderline and malignant phyllodes tumors, there was 100% agreement in 53% of cases (10/19) and 90% agreement in 79% of cases (15/19).
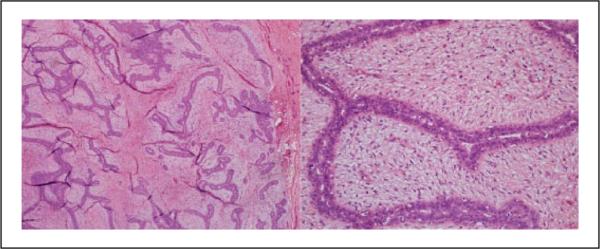
Figure 1.
Case 16: All 10 pathologists diagnosed this lesion as either fibroadenoma or cellular fibroadenoma (hematoxylin and eosin; 4× and 40×).
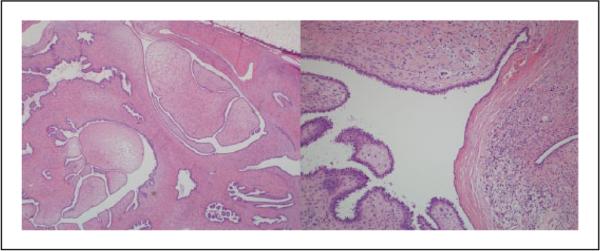
Figure 2.
Case 4: All 10 pathologists diagnosed this lesion as a phyllodes tumor, with 7 diagnosing it as borderline, 2 as malignant, and 1 as benign (hematoxylin and eosin; 4× and 20×).
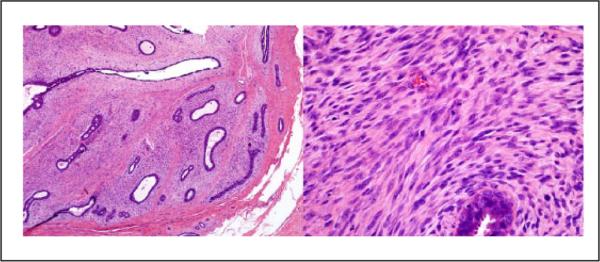
Figure 3.
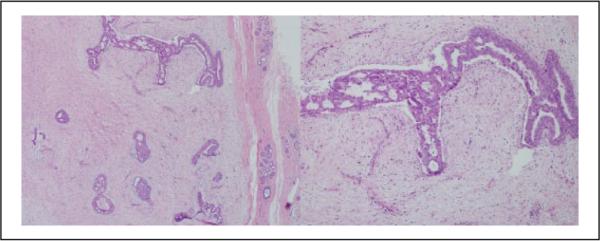
Case 1: This lesion was diagnosed cellular fibroadenoma by 5 pathologists and benign phyllodes tumor by 5 pathologists (hematoxylin and eosin; 4× and 40X×).
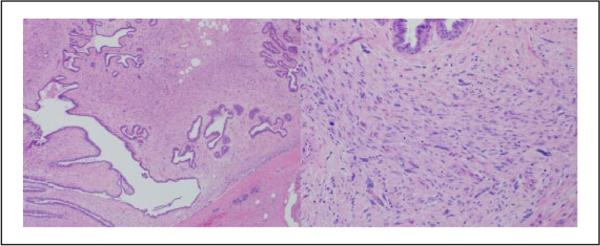
Figure 4.
Case 10: This lesion was diagnosed benign phyllodes tumor by 6 pathologists and fibroadenoma or cellular fibroadenoma by 4 pathologists (hematoxylin and eosin; 4× and 10×).
Figure 5.
Case 2: This lesion was diagnosed cellular fibroadenoma by 4 pathologists and borderline phyllodes tumor by 4 pathologists. The remaining diagnoses were benign phyllodes tumor and nodular pseudoangiomatous stromal hyperplasia (hematoxylin and eosin; 4× and 20×).
Figure 6.
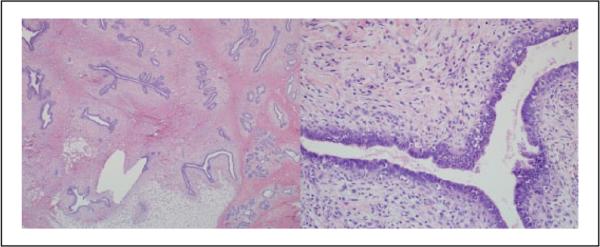
Case 9: This lesion was diagnosed benign phyllodes tumor by 6 pathologists, borderline phyllodes tumor by 3 pathologists, and cellular fibroadenoma by 1 pathologist (hematoxylin and eosin; 2× and 10×).
Discussion
The diagnostic distinction of fibroadenomas with cellular stroma (cellular fibroadenomas) and phyllodes tumors is based on the “best fit” within a set of 6 criteria established by the WHO.1 In most cases the diagnosis is straightforward. However, occasional cases with significantly overlapping features remain a challenge to classify as there is no single histologic feature unequivocally separating the two.3-5 This distinction usually involves deciding between a cellular fibroadenoma and a benign phyllodes tumor, but in our study nine of the 21 cases (43%) had diagnoses ranging from fibroadenoma to borderline phyllodes tumor. Thus, even pathologists who specialize in breast pathology disagreed significantly not only in separating fibrodenomas from benign phyllodes tumors but also in distinguishing the subclasses of phyllodes tumors in these challenging cases. Unfortunately, these distinctions are not without consequence, as the decision for further surgical treatment, particularly for the borderline and malignant phyllodes tumors, sometimes rests on these subjective criteria. In attempts to improve on pathologists’ ability to better distinguish types of fibroepithelial lesions, numerous studies have looked at using immunohistochemical markers, but to date none has proven to be useful in everyday practice and histology remains the current standard.6
If the criteria used to distinguish different types of fibroepithelial lesions remain subjective, and pathologists cannot agree on the various subclassifications, why do we continue to make these distinctions? The predominant reason is to determine the likelihood of a particular fibroepithelial lesion recurring and/or possibly metastasizing. Within phyllodes tumors, recurrence rates tend to increase from benign to borderline to malignant.4,5,7-9 For benign phyllodes tumors, recurrence rates are generally reported to be 20% or less.4,5 Recurrence rates as high as 17% have also been reported in fibroadenomas.5,10 Additionally, unlike for borderline or malignant phyllodes tumors, studies have suggested that additional surgery following a diagnosis of benign phyllodes tumor may not be necessary as recurrence rates were not significantly affected by margin status.8,11,12 This begs the following question: If the likelihood of recurrence is similar between a cellular fibroadenoma and a benign phyllodes tumor and additional surgery for clear margins may not be beneficial for either, why separate these 2 diagnoses? One possible reason is that there are studies that have identified benign phyllodes tumors recurring as borderline or malignant tumors and there are rare reports of benign phyllodes tumors metasta-sizing.9,13-20 However, a large review of the literature7 showed that the overall rate of histologic progression from a benign phyllodes tumor to a higher grade on average is very low (4%) and some of these studies commented that possibly due to inadequate tissue sampling, more “malignant” areas of the original tumor might not have been identified.14,16,17
Given the current literature, it seems there is minimal if any significant difference in clinical behavior between a cellular fibroadenoma and a benign phyllodes tumor in terms of likelihood of recurrence or progression to a higher-grade tumor. In our study, we found considerable agreement when cellular fibroadenomas and benign phyllodes tumors are distinguished from borderline and malignant phyllodes tumors. We agree with the proposal in the 2012 WHO Classification of Tumours of the Breast1 suggesting that, in difficult cases, a diagnosis of fibroepithelial lesion be rendered so as to avoid unnecessary additional surgical treatment and to convey that these lesions are currently not reproducibly distinguished by pathologists. Further studies are needed to focus on the establishment of criteria to better distinguish benign phyllodes tumors from borderline and malignant tumors, since this distinction is the one that appears to have clinical significance.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Tan PH, Tse G, Lee A, Simpson JF, Hanby AM. Fibroepithelial tumours. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van der Vijver MJ, editors. WHO Classification of Tumours of the Breast. IARC Press; Lyon, France: 2012. pp. 141–147. [Google Scholar]
- 2.Bellocq JP, Magro G. Fibroepithelial tumours. In: Tavassoli FA, Devilee P, editors. WHO Classification of Tumours: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press; Lyon, France: 2003. pp. 99–103. [Google Scholar]
- 3.Lee AH. Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast. Histopathology. 2008;52:45–57. doi: 10.1111/j.1365-2559.2007.02893.x. [DOI] [PubMed] [Google Scholar]
- 4.Giri D. Recurrent challenges in the evaluation of fibroepithelial lesions. Arch Pathol Lab Med. 2009;133:713–721. doi: 10.5858/133.5.713. [DOI] [PubMed] [Google Scholar]
- 5.Tan PH, Ellis IO. Myoepithelial and epithelial-myoepithelial, mesenchymal and fibroepithelial breast lesions: updates from the WHO Classification of Tumours of the Breast 2012. J Clin Pathol. 2013;66:465–470. doi: 10.1136/jclinpath-2012-201078. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Kandil D, Cosar EF, Khan A. Fibroepithelial tumors of the breast: pathologic and immunohistochemical features and molecular mechanisms. Arch Pathol Lab Med. 2014;138:25–36. doi: 10.5858/arpa.2012-0443-RA. [DOI] [PubMed] [Google Scholar]
- 7.Spitaleri G, Toesca A, Botteri E, et al. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol. 2013;88:427–436. doi: 10.1016/j.critrevonc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Kim JY, Kim do H, Jung WH, Koo JS. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat. 2013;141:353–363. doi: 10.1007/s10549-013-2684-x. [DOI] [PubMed] [Google Scholar]
- 9.Tan PH, Thike AA, Tan WJ, et al. Predicting clinical behavior of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol. 2012;65:69–76. doi: 10.1136/jclinpath-2011-200368. [DOI] [PubMed] [Google Scholar]
- 10.Organ CH, Jr, Organ BC. Fibroadenoma of the female breast: a critical clinical assessment. J Natl Med Assoc. 1983;75:701–704. [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan M, Argani P, Cimino-Mathews A. Benign and low-grade fibroepithelial neoplasms of the breast have low recurrence rate after positive surgical margins. Mod Pathol. 2014;27(2 suppl):42A. doi: 10.1038/modpathol.2015.157. [DOI] [PubMed] [Google Scholar]
- 12.Zurrida S, Bartoli C, Galimberti V, et al. Which therapy for unexpected phyllode tumor of the breast? Eur J Cancer. 1992;28:654–657. doi: 10.1016/s0959-8049(05)80119-4. [DOI] [PubMed] [Google Scholar]
- 13.Blichert-Toft M, Hansen JP, Hansen OH, Schiodt T. Clinical course of cystosarcoma phyllodes related to histologic appearance. Surg Gynecol Obstet. 1975;140:929–932. [PubMed] [Google Scholar]
- 14.Treves N, Sunderland DA. Cystosarcoma phyllodes of the breast: a malignant and a benign tumor; a clinicopathological study of seventy-seven cases. Cancer. 1951;4:1286–1332. doi: 10.1002/1097-0142(195111)4:6<1286::aid-cncr2820040614>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.West TL, Weiland LH, Clagett T. Cystosarcoma phyllodes. Ann Surg. 1971;173:520–528. doi: 10.1097/00000658-197104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lester J, Stout AP. Cystosarcoma phyllodes. Cancer. 1954;7:335–353. doi: 10.1002/1097-0142(195403)7:2<335::aid-cncr2820070219>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Reinfuss M, Mitus J, Duka K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996;77:910–916. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Chaeny AW, Pollack A, McNeese MD, et al. Primary treatment of cystosarcoma phyllodes of the breast. Cancer. 2000;89:1502–1511. doi: 10.1002/1097-0142(20001001)89:7<1502::aid-cncr13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Grimes MM. Cystosarcoma phyllodes of the breast: histo-logic features, flow cytometric analysis, and clinical correlations. Mod Pathol. 1992;5:232–239. [PubMed] [Google Scholar]
- 20.Hajdu SI, Espinosa MH, Robbins GF. Recurrent cystosarcoma phyllodes: a clinicopathologic study of 32 cases. Cancer. 1976;38:1402–1406. doi: 10.1002/1097-0142(197609)38:3<1402::aid-cncr2820380346>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]