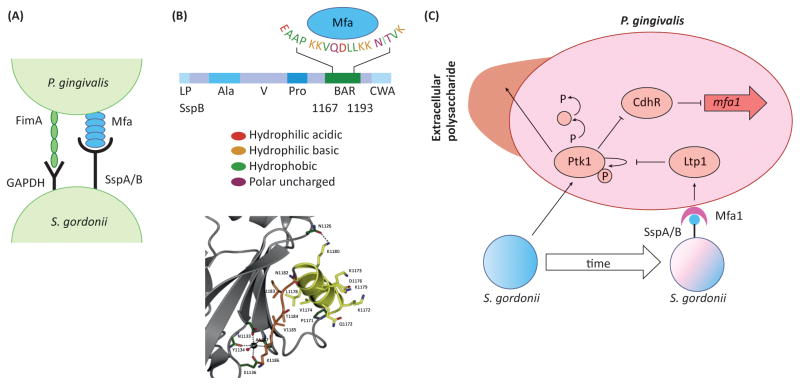
Figure 2. The molecular basis of synergy between P. gingivalis and S. gordonii.
A. The physical association between P. gingivalis and S. gordonii involves FimA–GAPDH and Mfa1–SspA/B adhesin–receptor pairs on the surfaces of the organisms. B. The upper panel shows the domain structure of the SspB protein and the amino acid residues involved in recognition of Mfa1. BAR spans aa residues 1167–1193, and the EAAP, KKVQDLLKK and NITVK sequences are involved in Mfa1 recognition. The lower panel shows the structure of the SspB C-terminal region with the protruding BAR domain stabilized by a calcium ion, and coordinated by three main chain and two side-chain oxygen atoms and a water molecule. Reproduced from [4] with permission. C. Signaling interactions between S. gordonii and P. gingivalis. S. gordonii induces autophosphorylation of the Ptk1 tyrosine kinase. Ptk1 activates a signaling cascade which converges on inactivation of the transcriptional repressor CdhR. As a result, expression of the minor fimbrial adhesin subunit Mfa1 is elevated and P. gingivalis is ‘primed’ for attachment to S. gordonii. Ptk1 is also a key component of the machinery for secretion of extracellular polysaccharide, and active Ptk1 increases the amount of polysaccharide material on the surface of P. gingivalis. Over time, however, direct contact mediated by Mfa1-SspA/B binding increases expression of the tyrosine phosphatase Ltp1, which dephosphorylates Ptk1, ultimately relieving repression of ChdR. Expression of the Mfa1 fimbrial adhesin is reduced and community development is constrained.
Abbreviations: Ala, alanine-rich repeats; BAR, SspB Adherence Region, the Mfa1-interacting domain; CWA, cell wall anchor; LP, leader peptide; Pro, proline-rich repeats; V, variable region.