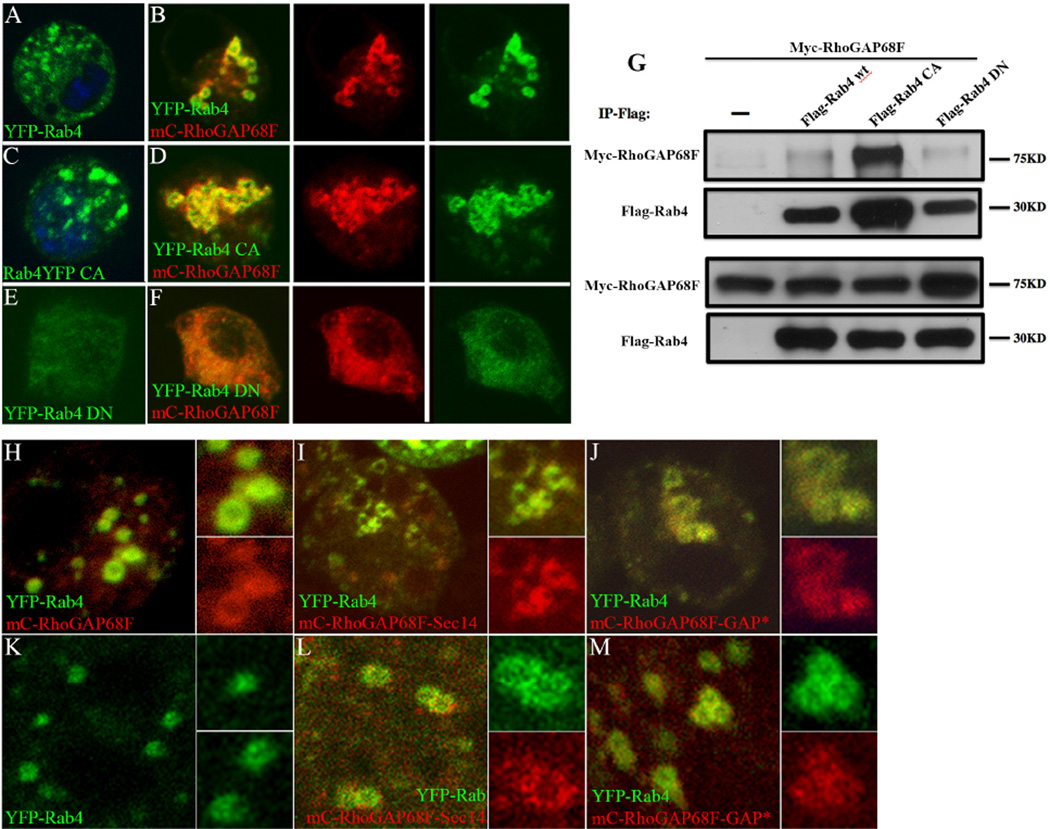
Figure 7.
RhoGAP68F interacts physically and preferentially with the active Rab4 protein and the RhoGAP68F–Sec14 domain localizes to Rab4 endosomes. (A–F) Localization and (G) physical interaction of RhoGAP68F with wild type, constitutively active and dominant negative YFP-Rab4 variants. (A) YFP-Rab4 localized to puncta in the cytoplasm. (B) Expression of mC-RhoGAP68F together with YFP-Rab4 caused clustering and enlargement of Rab4 endosomes. (C) Active YFP-Rab4 caused enlargement of the Rab4 endosomes, (D) Expression of mC-RhoGAP68F together with active YFP-Rab4 caused further enlargement and clustering of Rab4 endosomes and formation of extensive tubular structures. (E) Dominant negative YFP-Rab4 localized in a diffused pattern in the cytoplasm. (F) mC-RhoGAP68F expressed with the dominant negative Rab4 variant localized in a similar diffused pattern. (G) Co-IP assays revealed that the association of the RhoGAP68F protein was (~3.26 fold) stronger with the constitutively active Flag-Rab4 variant, and (~2.88 fold) weaker with the dominant negative variant compared to the association with the wild type tagged protein in a representative experiment. (H–M) Localization of mC-RhoGAP68F variants in S2 cells (H–J), and in vivo (K–M). A RhoGAP68F Sec14-only variant localized to Rab4 endosomes both in S2 cells and in vivo (Fig. 7I, 7L). Similarly, a GAP deficient RhoGAP68F localized to the Rab4 compartment in S2 cells and in vivo (Fig. 7J, 7M). Both variants caused mild clustering and enlargements of the YFP-Rab4 endosomes (Fig 7I–J, L–M compared to H–K).