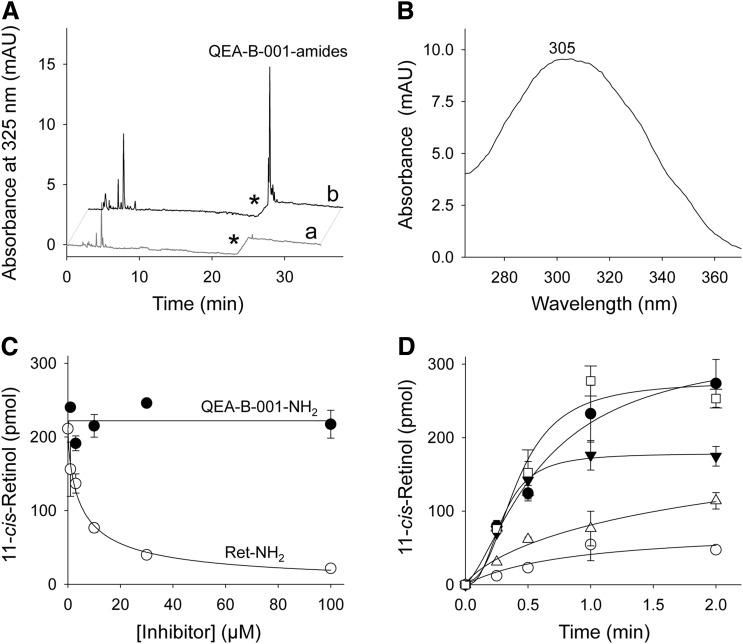
Fig. 3.
Amidation of QEA-B-001-NH2 and inhibition of RPE65. Primary amines were preincubated with bovine RPE microsomes at room temperature for 5 minutes; then all-trans-retinol was added and the mixture was incubated at 37°C. (A) HPLC chromatograph showing acylation of QEA-B-001-NH2 by LRAT in RPE microsomes; chromatograms “a” and “b” correspond to extracts of RPE microsomes in the absence and presence of QEA-B-001-NH2, respectively. Asterisks indicate a step change in the ethyl acetate mobile phase concentration (from 10 to 30% hexane). Under these chromatographic conditions, the free amine of QEA-B-001-NH2 did not elute from the normal-phase HPLC column without addition of ammonia to the mobile phase. (B) UV-Visible absorbance spectrum of a peak at 26 minutes of elution. This spectrum corresponds to QEA-B-001-NH2 amide. (C) Effect of inhibitor concentrations on the production of 11-cis-retinol. Inhibition of RPE65 enzymatic activity was measured as a decline in 11-cis-retinol production. ●, QEA-B-001-NH2; ○, retinylamine (Ret-NH2). All incubation mixtures were quenched by addition of methanol after 1 hour of incubation at room temperature. (D) 11-cis-Retinol production in the presence of 5 µM QEA-B-001-NH2 (●), 30 µM QEA-B-001-NH2 (▼), 5 µM Ret-NH2 (△), 30 µM retinylamine (○), and control (□).