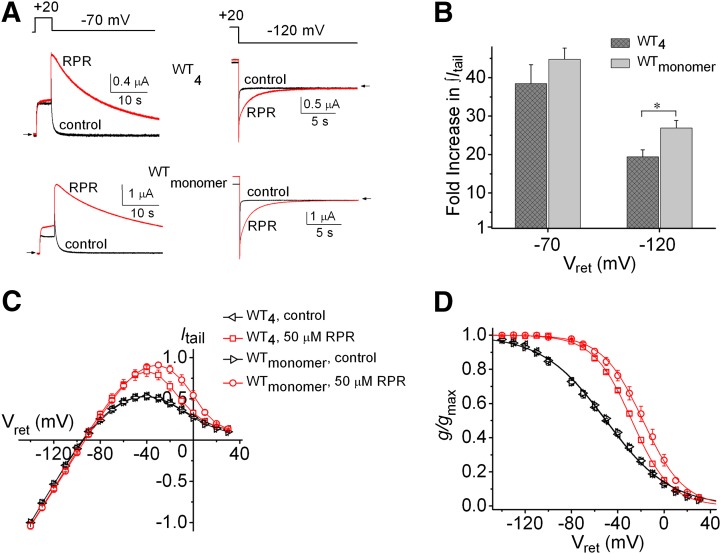
Fig. 1.
RPR exerts less potent effects on inactivation and deactivation of homotetrameric hERG1 concatemers (WT4) than WT hERG1 channels formed from monomers. (A) Effect of 50 µM RPR on WT4 and WTmonomer hERG1 channel currents elicited by a 30-second pulse to −70 mV (left panels) or an 18-second pulse to −120 mV (right panels). (B) Fold increase in ∫Itail for WT4 and WTmonomer hERG1 channels at indicated Vret; *P = 0.015. (C) Fully-activated Itail-Vret relationships in the absence and presence of 50 µM RPR for WT4 (n = 6) and WTmonomer (n = 7) hERG1 channels, respectively. Currents were normalized to Itail at −140 mV measured under control conditions. (D) Voltage dependence of recovery from inactivation for WT4 and WTmonomer channels in the absence and presence of 50 µM RPR. For WTmonomer channels, control: V0.5inact = −50.2 ± 1.4 mV; z = 0.91 ± 0.01; 50 µM RPR: V0.5inact = −18.4 ± 2.8 mV; z = 1.52 ± 0.08 (n = 7). For WT4 channels, control: V0.5inact = −51.6 ± 1.6 mV; z = 0.92 ± 0.02; 50 µM RPR: V0.5inact = −27.6 ± 0.8 mV; z = 1.48 ± 0.05 (n = 6). For (C and D), relationships for WT4 and WTmonomer channels in the presence of 50 µM RPR were significantly different from one another (P < 0.0001, two-way analysis of variance).