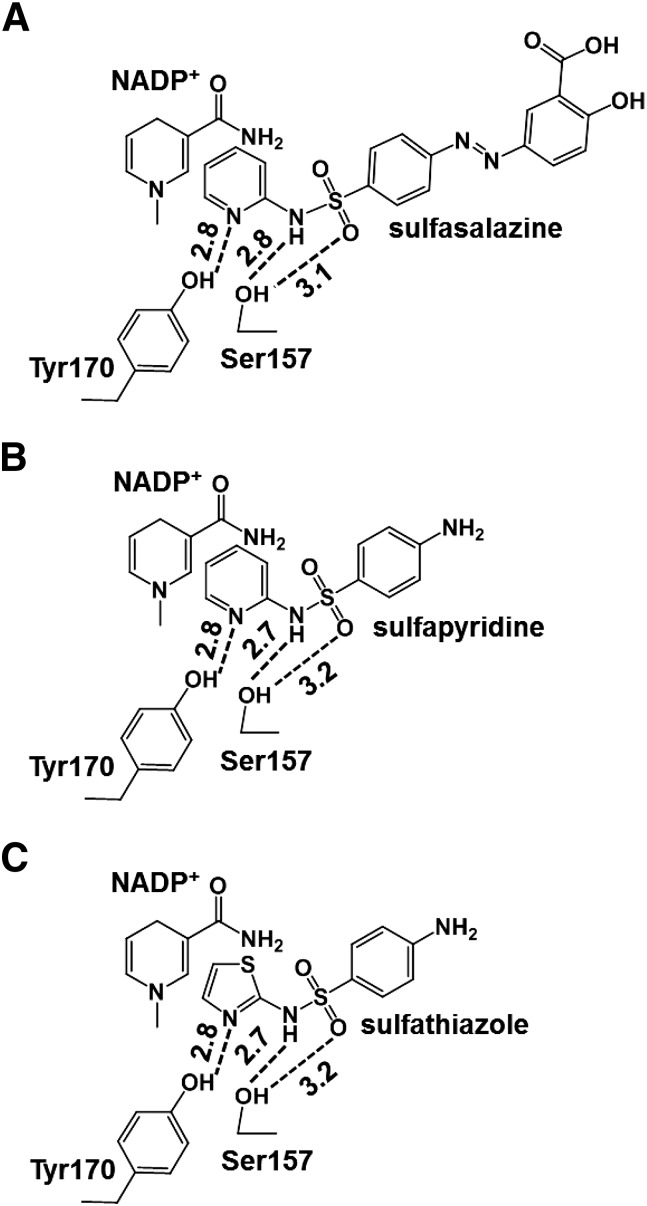
Fig. 9.
Interaction of the sulfa drugs with active site residues and NADP+ in human SPR. The ternary complexes of human SPR with NADP+ (nicotinamide moiety) and sulfasalazine (A), sulfapyridine (B), or sulfathiazole (C) are derived from their reported crystal structures in the Protein Data Bank. The sulfa drugs are anchored by hydrogen bonding to catalytic residues tyrosine 170 and serine 157 and hydrophobic and/or π-electron interactions of the pyridine or thiazole rings in the sulfa drugs with the nicotinamide group of the NADP+ cofactor. The distances between the nicotinamide of NADP and pyridine or thiazole ring of sulfa drugs are around 3.2–4.0 Å. Hydrogen bonds are shown by broken lines, and the corresponding distances (Å) are indicated.