ABSTRACT
A common stress encountered by both pathogenic and environmental bacteria is exposure to a low-pH environment, which can inhibit cell growth and lead to cell death. One major defense mechanism against this stress is the arginine deiminase (ADI) pathway, which catabolizes arginine to generate two ammonia molecules and one molecule of ATP. While this pathway typically relies on the utilization of arginine, citrulline has also been shown to enter into the pathway and contribute to protection against acid stress. In the pathogenic bacterium Streptococcus pyogenes, the utilization of citrulline has been demonstrated to contribute to pathogenesis in a murine model of soft tissue infection, although the mechanism underlying its role in infection is unknown. To gain insight into this question, we analyzed a panel of mutants defective in different steps in the ADI pathway to dissect how arginine and citrulline protect S. pyogenes in a low-pH environment. While protection provided by arginine utilization occurred through the buffering of the extracellular environment, citrulline catabolism protection was pH independent, requiring the generation of ATP via the ADI pathway and a functional F1Fo-ATP synthase. This work demonstrates that arginine and citrulline catabolism protect against acid stress through distinct mechanisms and have unique contributions to virulence during an infection.
IMPORTANCE An important aspect of bacterial pathogenesis is the utilization of host-derived nutrients during an infection for growth and virulence. Previously published work from our lab identified a unique role for citrulline catabolism in Streptococcus pyogenes during a soft tissue infection. The present article probes the role of citrulline utilization during this infection and its contribution to protection against acid stress. This work reveals a unique and concerted action between the catabolism of citrulline and the F1Fo-ATPase that function together to provide protection for bacteria in a low-pH environment. Dissection of these collaborative pathways highlights the complexity of bacterial infections and the contribution of atypical nutrients, such as citrulline, to pathogenesis.
INTRODUCTION
Adaptation to environmental acidification presents a significant challenge to microorganisms, including both pathogenic and environmental bacterial species (1). Due to the near ubiquitous nature of this stress, elucidation of adaptive strategies and their associated molecular mechanisms has broad implications for our understanding of both bacterial physiology and virulence. One of the most widely used bacterial mechanisms for protection against acid stress involves the catabolism of arginine via the arginine deiminase (ADI) pathway (2–4). However, each of the various components of this pathway can be adapted in several different ways to promote survival in acidic environments. Therefore, the challenge becomes understanding how the ADI pathway has been adapted in an individual bacterial species.
In the Gram-positive pathogen Streptococcus pyogenes (group A streptococcus), it has recently been shown that the ADI pathway metabolite citrulline makes an unexpected arginine-independent contribution to both colonization and virulence (5). This human pathogen is responsible for a large number of diseases that range in severity and invasiveness (6). Common, noninvasive soft tissue infections include bacterial pharyngitis and impetigo, in addition to the less common but invasive and often life-threatening necrotizing fasciitis and immune-pathological syndromes like rheumatic fever (6). It was recently discovered that mutations that blocked the ability of S. pyogenes to catabolize arginine attenuated virulence in a murine model of soft tissue infection (5). However, mutants that block catabolism of citrulline resulted in hyperattenuation (5), revealing an unexpected tissue-specific and arginine-independent role for citrulline metabolism in pathogenesis. The molecular basis for this contribution of citrulline catabolism to pathogenesis is unclear.
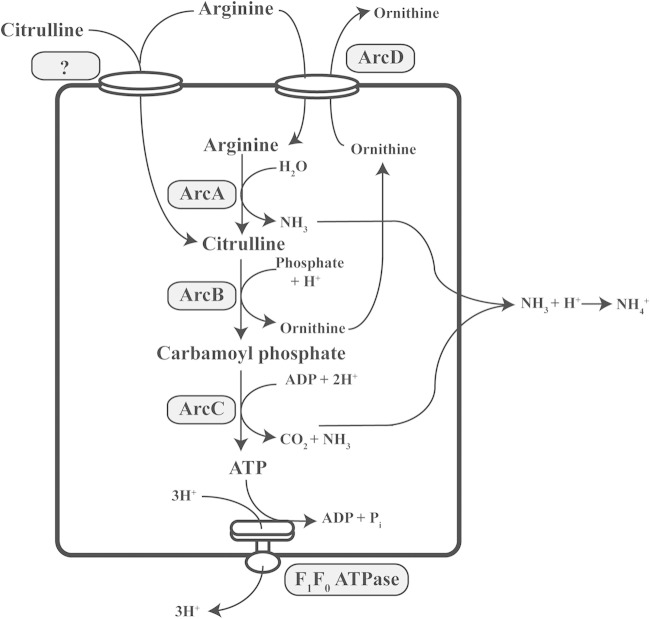
The ADI pathway in S. pyogenes is composed of three enzymes: ArcA, ArcB, and ArcC, which localize to the cytoplasm of the bacteria, and ArcD, a membrane-embedded protein involved in the transport of arginine (7–9). These proteins function together to generate three products: ATP, a molecule of ammonia, and a molecule of carbon dioxide (Fig. 1). The ability of this pathway to generate an ATP molecule along with two protective ammonia molecules may explain its wide distribution among the genomes of both Gram-negative and Gram-positive bacterial species. Significantly, the ADI pathway is ubiquitous in the genomes of the Gram-positive lactic acid bacterial species, including all S. pyogenes genomes sequenced to date.
FIG 1.
Arginine and citrulline catabolism in S. pyogenes and its coordination with the F1Fo-ATPases. Catabolism of arginine and citrulline occurs through the multienzyme arginine deiminase pathway and involves the transport of arginine through the antiporter ArcD and an unknown transporter, followed by catabolism via the enzymes ArcA, ArcB, and ArcC. Catabolism of arginine produces two molecules of ammonia and one molecule of ATP. Catabolism of citrulline can produce one molecule of ammonia and one molecule of ATP. The F1Fo-ATPase can export three protons outside the cell with the concomitant hydrolysis of ATP to ADP.
A defining characteristic of the many different and divergent species that comprise the group lactic acid bacterial (family Lactobacillacea) is that their metabolisms produce a common metabolic end product, lactic acid (10). Associated with this, lactic acid bacteria lack a functional electron transport chain and rely upon glycolysis for the generation of energy for growth. The production of lactic acid and/or other organic acids is necessary for the essential reoxidation of the NADH cofactor (10) and generally results in significant autoacidification of their surrounding environment. Consistent with this, analysis of transcriptome profiles of S. pyogenes during growth in murine soft tissue revealed a profile closely resembling that of the bacterium growing in an autoacidified low-pH environment in vitro (11). Thus, acid stress is an intrinsic consequence of the S. pyogenes lactic acid metabolism that acts to remodel its own niche as it grows in tissue. This inevitable exposure to a low-pH environment suggests that S. pyogenes must have a robust mechanism for adaptation to acid stress.
It is then not surprising that analysis of the ADI pathway's role in S. pyogenes pathogenesis revealed that it contributes to virulence in murine models of colonization and soft tissue infection (5). One important role for the ADI pathway involves modulation of the host's innate immune response as arginine is a critical substrate for the pathway of nitric oxide generation by inflammatory cells (12, 13). While S. pyogenes uses the ADI pathway to deplete arginine, rendering it unavailable to host cells (5), arginine depletion cannot entirely account for the ADI pathway's contribution to virulence. The loss of any of the enzymes downstream of ArcA in the pathway resulted in hyperattenuation for disease, and this defect in virulence was greater than could be accounted for on the basis of ArcA-mediated depletion of arginine. Analysis of multiple mutants revealed that hyperattenuation did not result from the accumulation of a toxic intermediate product but rather was associated with a requirement for the utilization of citrulline. Compared to arginine, citrulline lacks one of the two ammonia groups necessary for protection against acid stress: thus, the contribution of citrulline catabolism to pathogenesis remains unclear.
In the present work, we examined the mechanism by which the arginine-independent catabolism of citrulline via the ADI pathway protects S. pyogenes from acid stress. Analysis of several Arc mutants allowed a high-resolution dissection of the process by which arginine and citrulline catabolism protects S. pyogenes from acid stress. This work elucidates the unexpected role for citrulline catabolism in virulence and reveals the generation of ATP and ammonia to be an essential function of the ADI pathway in adaption to an acidic environment. Taken together, these data illustrate the multiple strategies by which the ADI pathway has been adapted by S. pyogenes for protection from acid stress in order to promote its growth and pathogenesis in soft tissue.
MATERIALS AND METHODS
Streptococcus pyogenes strains, media, and growth conditions.
All experiments utilized the Streptococcus pyogenes strain HSC5 (14, 15) and mutant derivatives of this strain described elsewhere (5). Culture of S. pyogenes for the acid stress assay and routine growth was in Todd-Hewitt medium (Difco) supplemented with 0.2% yeast extract (Difco) (THY medium). When indicated, strains were cultured in C-medium (0.5% protease peptone 3 [Difco], 1.5% yeast extract [Difco], 10 mM K2HPO4, 0.4 mM MgSO4, 17 mM NaCl). Supplementation of medium with amino acids involved addition of a filter-sterilized solution of either 1 M arginine or 0.5 M citrulline, to the designated concentration. All growth experiments were performed in sealed culture tubes placed at 37°C under static conditions. Solid medium was made by the addition of 1.4% Bacto agar (Difco) to the medium and was cultured anaerobically in sealed jars in the presence of gas-generating packets (GasPak catalog no. 70304 BBL). When necessary, chloramphenicol was added at the concentration of 3 μg/ml.
Acid stress assay.
The indicated strains were grown at 37°C in 10 ml of unmodified THY medium or THY medium supplemented with 10 mM arginine or citrulline. At selected time points, a 100-μl aliquot from a resuspended culture was removed, serial dilutions were prepared in phosphate-buffered saline (PBS), and aliquots were plated on THY medium for determination of CFU, which were enumerated following 24 h of incubation. At the end of the 6-day period, bacterial cells were removed from 5-ml samples by centrifugation, and the supernatants were sterilized by filtration. The pH of the medium was then measured using a pH meter (Accumet AB15).
Measurement of ammonia production.
Overnight cultures were back-diluted into 50 ml of THY medium and grown to the exponential phase. The bacterial cells were then collected by centrifugation, washed with citrate phosphate buffer (pH 6.0), and resuspended in the same buffer to an optical density at 600 nm (OD600) between 1.0 and 2.0. Arginine was added to a final concentration of 10 mM, a sample was removed for the 0-h time point, and the cultures were incubated at 37°C for 1 h. Cells were then collected by centrifugation, and a small aliquot of the supernatant was removed. The concentration of ammonia was determined by an NADP-linked enzymatic assay (ammonia assay kit; Sigma catalog no. AA0100) according to the manufacturer's instructions. Values reported were corrected by subtraction of the 0-h time point and normalized relative to the final culture density (OD600).
Conditioned medium assay.
The ability of conditioned medium to protect the ΔArcB mutant from acid stress was assessed as follows: conditioned medium from each tested strain was first generated by growth in THY medium in a sealed culture tube at 37°C for 6 days. The streptococcal cells were then removed by centrifugation, the pH of the medium was measured, and the solution was sterilized by filtration through a 0.2-μm-pore filter. The ΔArcB mutant was prepared by overnight growth in 50 ml of THY medium. Cells were collected by centrifugation, washed twice in 10 ml of PBS, and then resuspended in 1 ml of PBS. Aliquots of this suspension were added to 1 ml of the designated conditioned medium to reach a final concentration of approximately 107 CFU/ml. The suspension was then incubated at 37°C, and viability was determined by measuring CFU on days 0, 3, and 6.
DCCD sensitivity assay.
To measure the influence of N,N′-dicyclohexylcabodiimide (DCCD) on protection in unsupplemented medium, the designated strains were initially grown in unsupplemented THY medium for 24 h, and aliquots were used to inoculate fresh THY medium supplemented with various concentrations of DCCD (Sigma catalog no. D8002) to a density of approximately 108 CFU/ml. These cultures were incubated for an additional 3 days, and the number of CFU was determined. To determine protection by arginine and citrulline, growth from overnight cultures in unsupplemented THY medium was used to inoculate fresh THY medium containing arginine or citrulline (10 or 20 mM). These cultures were incubated at 37°C for 24 h, at which time DCCD was added to 1.0 mM. Reference cultures received an equivalent volume of vehicle (ethanol). Cultures were incubated at 37°C for an additional 5 days, and the number of CFU was determined.
Statistical analyses.
For all data, the mean values determined were tested for significance using the Mann-Whitney U test. Computation of the statistics test was performed with the Graphpad software (San Diego, CA) Prism (version 6). For all tests, the null hypothesis was rejected for a P value of <0.05.
RESULTS
Arginine and citrulline metabolism via the ADI pathway protects S. pyogenes from acid stress.
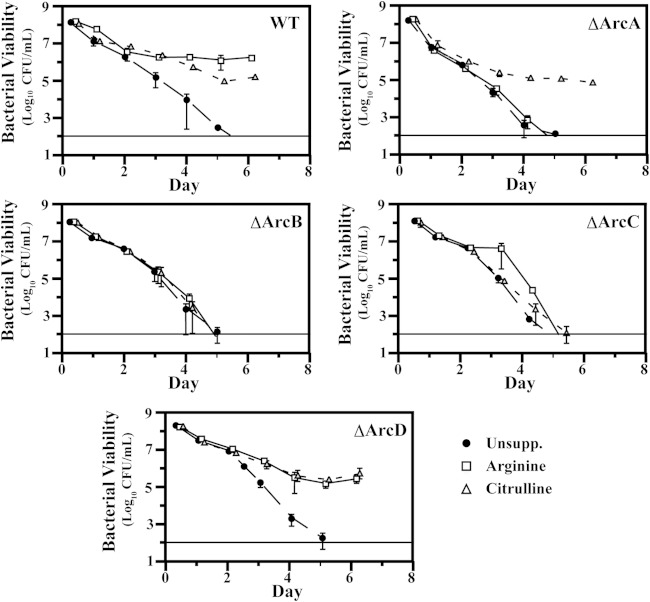
To probe the relationship between virulence and protection of S. pyogenes against acid stress, we examined a previously developed generated panel of ADI pathway mutants (5) to dissect how arginine and citrulline catabolism can protect bacteria from acid stress and to assess the individual contributions of ammonia and ATP to protection. To mimic the acid stress commonly encountered by bacteria in the environment, S. pyogenes was cultured in a glucose-rich medium (THY) whose pH is rapidly acidified due to the accumulation of the fermentation by-product lactic acid (16). Growth in this medium results in a rapid decrease in culture pH as the bacteria reach a high culture density (see Fig. S1 in the supplemental material). Following 6 days of growth under these conditions, the pH of cultures decreases from an initial value of 7.5 to pH 5.4 (Fig. 2A). Assessment of the viability of the wild-type (WT) strain in unsupplemented medium over this period by CFU enumeration revealed a gradual decrease in CFU, with no bacteria detected above the limit of detection on day 6 (“WT” in Fig. 3). In contrast, when S. pyogenes cells are cultured in medium that lacks fermentable glucose and thus maintains a pH close to neutral, analysis of viability revealed only a slight decrease in CFU on day 6 for the WT and all Arc mutants (see Fig. S2 in the supplemental material), confirming that the loss of viability over time is due to the acidic environment generated by fermentation. Consistent with previously published results (17), supplementation of WT cultures with 10 mM arginine mitigated the decrease in bacterial numbers observed in unbuffered medium, with approximately 106 CFU remaining on day 6 (Fig. 3). This protection was dependent on a functional ADI pathway, as arginine did not protect cultures of mutants lacking arcA, -B, or -C (the ΔArcA, ΔArcB, and ΔArcC mutants, respectively). These strains exhibited decreased viability at a rate similar to that in unsupplemented medium, with CFU decreasing by at least 7 logs to below the limit of detection by day 6 (Fig. 3). An exception was observed upon mutation of arcD, as the resulting mutant (ΔArcD phenotype) could utilize arginine for protection from acid stress, with approximately 105 CFU remaining on day 6 (Fig. 3). This result was unexpected given that our previous work has demonstrated the inability of this mutant to utilize arginine as an energy source (5). Supplementation with 10 mM citrulline could also protect the WT, and protection was abrogated in the absence of arcB and -C, with both ΔArcB and ΔArcC mutants losing viability at a rate similar to that in unsupplemented medium (Fig. 3). Citrulline did protect the ΔArcA mutant, as citrulline enters the ADI pathway downstream of the arginine deiminase (Fig. 1), and the ΔArcA mutant was protected to the same extent as the WT, with at least 105 CFU maintained through day 6 (Fig. 3). Similar to arginine, the ΔArcD mutant maintained viability when supplemented with citrulline (Fig. 3). Taken together, these data demonstrate the contributions of the various ADI pathway genes to protection against acid stress for S. pyogenes. The observation that this protection did not require the ArcD antiporter suggests that arginine and citrulline can enter the cell via an alternate transporter(s).
FIG 2.
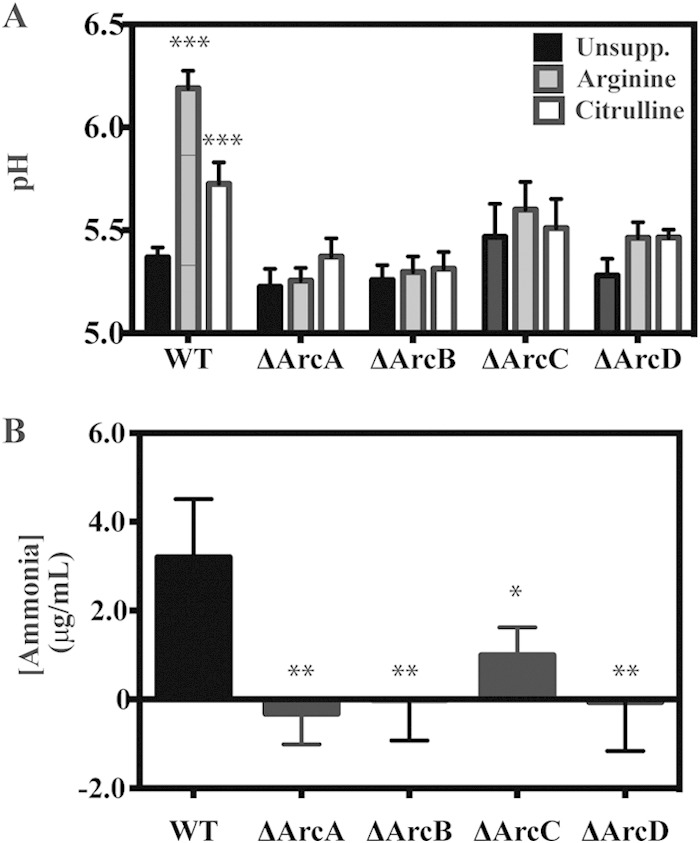
Influence of arginine and citrulline catabolism on medium pH and ammonia production. (A) Wild-type bacteria and the indicated strains were grown in THY medium supplemented with nothing (Unsupp.), 10 mM arginine, or 10 mM citrulline. Following 6 days of growth, the cultures were filter sterilized, and the pH of medium was measured using a pH probe. (B) Wild-type bacteria and the indicated strains were collected by centrifugation, resuspended in citrate buffer plus 10 mM arginine, and normalized to an OD600 of ∼2.0. The concentration of ammonia was measured spectroscopically after a 1-h incubation at 37°C. Data are presented as the mean and standard deviation from at least two independent experiments. Differences were tested for significance within the designated strains using the Mann-Whitney U test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
FIG 3.
Deletion of arc genes disrupt ability to utilize arginine and citrulline for protection against acid stress. Wild-type bacteria and the indicated strains were cultured in unsupplemented THY medium (Unsupp.), THY medium plus 10 mM arginine, or THY medium plus 10 mM citrulline for a 6-day period. At the indicated times, the cultures were resuspended, and a small aliquot was removed and serially diluted in PBS, followed by plating on solid medium for enumeration. The data displayed are the mean of the recovered CFU from at least three independent experiments.
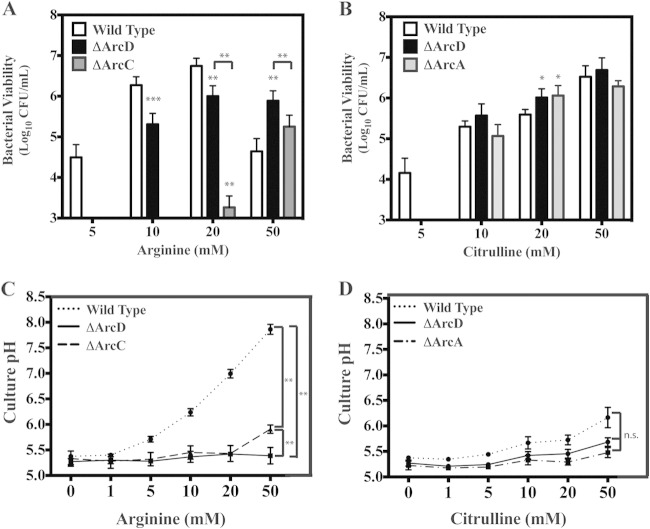
Arginine and citrulline protect in a dose-dependent manner.
The concentration dependence of arginine and citrulline protection was measured to compare their relative efficiencies in the WT. To compare to the wild type, all mutants were tested for their ability to utilize arginine and citrulline at a range of concentrations, the highest being 50 mM. However, only mutants that displayed some degree of protection when supplemented with either arginine or citrulline are discussed below. Protection was examined on day 6 since supplementation provided the largest degree of protection versus unsupplemented medium for the WT strain at this time point (approximately 6 logs). In this analysis, supplementation with as little as 5 mM arginine (Fig. 4A) or citrulline (Fig. 4B) provides over 4 logs of protection. Increasing citrulline concentrations provide a concomitant increase in protection, peaking at the highest concentration tested (50 mM) (Fig. 4B). In contrast, the protection provided for the wild type by arginine peaked at 20 mM at approximately 6 logs and then dropped by almost 2 logs when tested at 50 mM (Fig. 4A). This drop-off can be attributed to the increase in culture pH at this concentration (Fig. 4C), which is not observed for citrulline (Fig. 4D). Utilization of citrulline by the ΔArcA and ΔArcD mutants was found to be comparable to that of the WT, with the exception that no protection was observed at 5 mM citrullline (Fig. 4B). Analysis of the ΔArcD mutant revealed no protection by 5 mM arginine, significantly less protection than that of the wild type at 10 and 20 mM, and more protection at 50 mM (Fig. 4A). These data suggest that while transport of arginine can occur independently of ArcD, it is less efficient. Interestingly, while no protection over the limit of detection was observed for the ΔArcA or ΔArcB mutant with increasing concentrations of arginine (data not shown), the ΔArcC mutant was protected by high concentrations of arginine (20 and 50 mM) (Fig. 4A), although at a level significantly less than for the ΔArcD mutant (Fig. 4A). The finding of protection for the ΔArcC mutant at higher concentrations of citrulline was not observed (data not shown), suggesting protection required the ammonia molecule liberated from arginine during the generation of citrulline. Furthermore, it is likely that while both the ΔArcB and ΔArcC mutants can generate a molecule of ammonia during catabolism of arginine (Fig. 1), the ΔArcB mutant is incapable of producing a key by-product of the ADI pathway, ornithine, and thus is unable to transport arginine into the cell at a relatively high rate compared to the ΔArcC mutant. Thus, given the inability of an arcC mutant to convert carbamoyl phosphate to ATP (Fig. 1), protection from acid stress afforded by arginine catabolism in this mutant should be entirely dependent upon the production of ammonia by ArcA (Fig. 1) rather than ATP. Comparison of protection revealed the ΔArcD mutant was more efficient than the ΔArcC mutant in utilizing arginine; however, the protection observed for any mutant was less than that for the WT, indicating that all ADI proteins are required for the most efficient level of protection.
FIG 4.
Arginine and citrulline dose-dependent protection against acid stress does not directly correlate with culture pH. Wild-type bacteria and the indicated strains were cultured in THY medium supplemented with various concentrations of arginine (A and C) or citrulline (B and D) for a 6-day period. Following the 6-day period, the cultures were resuspended and an aliquot was removed and serially diluted in PBS, followed by plating on solid medium for enumeration of CFU. The cultures were then filter sterilized, and the culture pH was measured using a pH probe. The data displayed are the mean and standard deviation of recovered CFU and measured culture pH from at least three independent experiments. Differences in CFU and culture pH were tested for significance against the wild type or between the designated strains using the Mann-Whitney U test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Protection does not always correlate with culture pH.
As described above, catabolism of arginine by the WT strain results in the production of ammonia and a gradual increase in the medium pH as the bacteria enter the stationary phase (Fig. 1; see Fig. S1 in the supplemental material). Since several of the ADI pathway mutants (phenotypes ΔArcA, -C, and -D) retained the ability to utilize arginine and/or citrulline for protection against acid stress, although to a lesser degree than the wild type, it was of interest to probe the mechanism of this resistance by examining the culture pH following the 6-day growth period. For the WT strain, addition of 10 mM arginine and, to a lesser extent, citrulline significantly mitigated the decrease in culture pH compared to unmodified medium (Fig. 2A and Fig. 4C and D). This ability to modulate pH was dependent on ArcA, -B, -C, and -D, as the mutants lacking these enzymes lost the ability to alter the pH compared to unsupplemented medium (Fig. 2A and Fig. 4C and D). Consistent with this, all of the mutants had a significant decrease in ammonia production compared to the WT (Fig. 2B). However, supplementation with arginine and citrulline could protect the ΔArcA and ΔArcD mutants (described above). Thus, this mechanism of survival is independent of the modulation of culture pH. Examination over a range of arginine concentrations revealed a dose-dependent increase in the culture pH for the WT strain (Fig. 4C), which occurred to a lesser extent with citrulline (Fig. 4D). In contrast, the culture pH of the ΔArcD mutant remained relatively unchanged (Fig. 4C), despite the significant increase in protection that accompanied higher arginine or citrulline concentrations. A similar pattern was observed for the ΔArcA mutant with increasing amounts of citrulline (Fig. 4D). Analysis of the ΔArcC mutant revealed that while supplementation with 20 and 50 mM arginine provided some protection, a modest increase in culture pH was observed only at 50 mM arginine (Fig. 4D). Taken together, these data support the conclusion that protection in these mutants does not always correlate with culture pH.
Protection of the ΔArcC and ΔArcD mutants against acid stress occurs by two distinct mechanisms.
To further probe the relationship between arginine utilization, culture pH, and protection, the viability of the WT, ΔArcC, and ΔArcD strains was examined at a higher level of resolution with respect to the concentration of arginine added. As expected, there was a linear correlation between arginine concentration and culture pH for the WT strain (Fig. 5B). However, while the culture pH remained unchanged for the ΔArcD mutant, there was linear increase in pH for the ΔArcC mutant at concentrations above 20 mM (Fig. 4A and 5B). Analysis of viability revealed a difference in the patterns of protection between the ΔArcD and ΔArcC strains with respect to increasing arginine concentrations. For the ΔArcD strain, detectable protection was observed at a lower concentration of arginine than the ΔArcC strain, which rapidly increased to a maximum level at 20 mM arginine (Fig. 5A). No protection at 20 mM arginine was observed for the ΔArcC mutant, and protection increased in a linear pattern to reach a maximum at the highest concentration of arginine tested, 50 mM (Fig. 5A). Interestingly, when the relationship between viability and culture pH was examined (Fig. 5C), the wild type and ΔArcC mutant behaved similarly, suggesting a common mechanism of protection. In contrast, the relationship between the ΔArcD mutant's viability and culture pH (Fig. 5C) was not correlated in any discernible way, suggesting a unique mechanism of protection. Taken together, the different patterns of pH change and viability with respect to increasing arginine concentrations suggest that arginine can provide protection from acid stress by both pH-independent (ΔArcD mutant) and pH-dependent (ΔArcC mutant) mechanisms, with arginine utilization at these concentrations protecting wild-type bacteria primarily through a pH-dependent mechanism.
FIG 5.
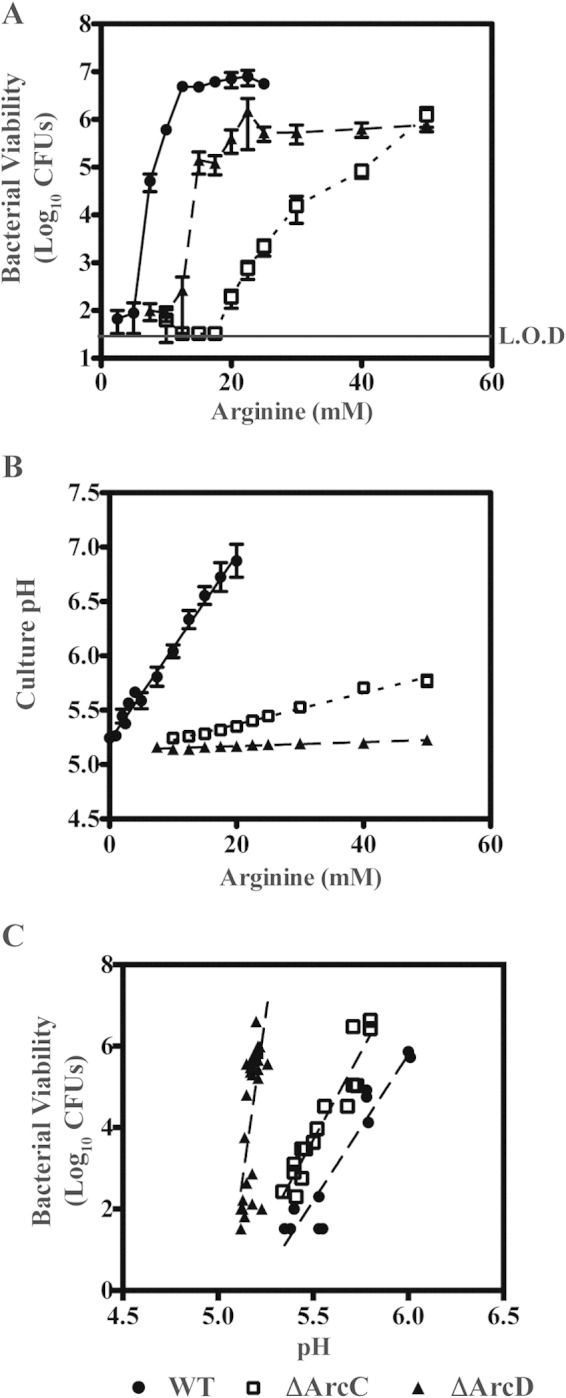
Arginine catabolism protection can occur by a pH-dependent mechanism and a pH-independent mechanism. (A) Wild-type bacteria (circles) and the ΔArcC (squares), and ΔArcD (triangles) mutants were cultured in THY medium supplemented with various concentrations of arginine for a 6-day period. Following the 6-day period, the viability of the cultures was measured as previously described. (B) The remaining culture was then filtered sterilized, and the pH was measured using a pH probe. (C) The relationships between viability and pH were investigated by replotting the data presented in panels A and B. The data displayed are the mean and standard deviation of recovered CFU from culture pH values of between 5.0 and 6.0 from at least three independent experiments. The level of detection (L.O.D.) was 33 CFU.
pH-independent protection in the ΔArcD mutant.
To further probe the mechanism of protection against acid stress, the viability of the ΔArcB mutant was examined in conditioned medium produced by the ΔArcD or ΔArcC mutant. Since the ΔArcB mutant is unable to utilize arginine for protection against acid stress, with no recovered CFU following 6 days of culture in THY medium supplemented with 50 mM arginine (data not shown), any observed protection of the ΔArcB mutant will be a function of the conditioned medium. To perform this analysis, approximately 107 CFU of the ΔArcB mutant were resuspended in conditioned medium from WT, ΔArcD, or ΔArcC cells that had been grown in various concentrations of arginine for 6 days. Viability of the suspensions was then monitored over an additional 6-day period and compared to that with resuspension of ΔArcB cells in medium conditioned by WT cells grown for 6 days in unsupplemented medium (average pH of 5.27). For the latter condition, enumeration of ΔArcB mutant CFU as a function of time revealed a steady decrease in viability, with no measurable CFU detected on day 6 (Fig. 6). In contrast, medium conditioned by the WT (10 mM arginine, average pH of 6.15) or the ΔArcC mutant (50 mM arginine, average pH of 5.94) protected ΔArcB mutant viability, with >105 CFU and 103 CFU recoverable on day 6, respectively (Fig. 6). Medium conditioned by the ΔArcD mutant (50 mM arginine, average pH of 5.28) did not protect, and viability decreased at a rate similar to that for unsupplemented WT-conditioned medium (Fig. 6). Since both the ΔArcD and ΔArcC mutants themselves are protected by arginine, this differential effect of conditioned medium differentiates the mechanism by which the ΔArcC and ΔArcD mutants utilize arginine catabolism to protect against acid stress. Whereas the ΔArcC mutant's protection is associated with an increased medium pH, the ΔArcD mutant's protection cannot be transferred in conditioned medium, suggesting this protection occurs in the interior of the bacterial cell.
FIG 6.
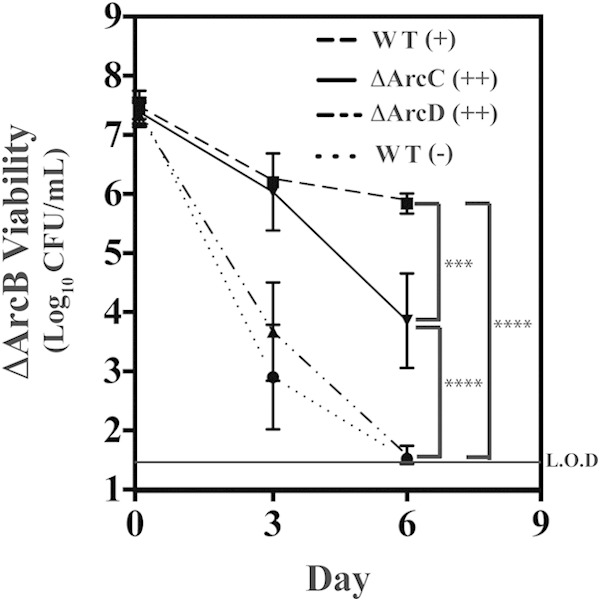
The ΔArcD mutant's protection against acid stress is not transferable in conditioned medium. The wild type and the indicated strains were cultured for 6 days in THY medium alone (−), THY medium plus 10 mM arginine (+), or THY medium plus 50 mM arginine (++). The cultures were then filter sterilized, and the conditioned medium was used to resuspend the ΔArcB mutant to a density of ∼107 CFU, which was placed back at 37°C. At the indicated time points, the cultures were resuspended, and the viability was measured as previously described. The data displayed are the mean and standard deviation of recovered CFU from at least three independent experiments. Difference in CFU were tested for significance against the wild type or between the designated strains using the Mann-Whitney U test (***, P < 0.001; ****, P < 0.0001). The level of detection (L.O.D.) was 33 CFU.
Protection of the ΔArcD mutant is sensitive to the ATPase inhibitor DCCD.
ATP is another key product of arginine catabolism by the ADI pathway (Fig. 1). Given that the protection of the ΔArcD mutant by arginine occurs without modulation of the medium's pH, it is possible that ATP plays a central role in this protective mechanism. Previous work has demonstrated that the coupling of ATP hydrolysis to the extrusion of protons by the F1Fo-ATPase is a major mechanism used by most lactic acid bacterial species for maintenance of intracellular pH homeostasis in acidic environments (18). The contribution of this mechanism to protection can be assessed using the F1Fo-ATPase inhibitor DCCD (19). Examination of the effect of increasing concentrations of DCCD on the WT and ΔArcC mutant showed no inhibition of arginine protection, as determined by recoverable CFU at any concentration of DCCD tested (Fig. 7). However, for the ΔArcD mutant, DCCD concentrations above 0.1 mM DCCD resulted in a measurable decrease in CFU, with a significant decrease apparent at 1.0 mM DCCD (Fig. 7). This differential sensitivity to DCCD indicates that protection in the ΔArcC and ΔArcD mutants occurs by different mechanisms and that protection of the ΔArcD mutant is dependent on the F1Fo-ATPase.
FIG 7.
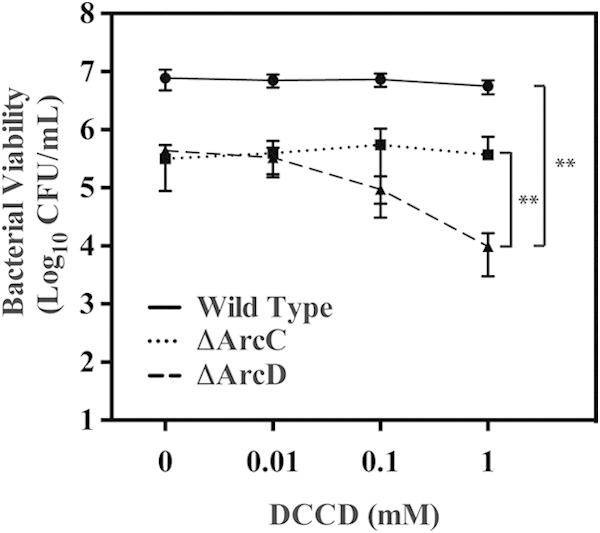
Protection of the ΔArcD mutant against acid stress is sensitive to DCCD. Wild-type bacteria were cultured in THY medium plus 10 mM arginine and the ΔArcC and ΔArcD mutants were cultured in THY medium plus 50 mM arginine for 24 h. Following this period, the culture was separated into aliquots, the designated concentration of DCCD was added, and the culture was allowed to incubate for an additional 3 days. After the 3-day period, the viabilities of the cultures were measured as previously described. The data displayed are the mean and standard deviation of recovered CFU from at least three independent experiments. Differences in CFU were tested for significance against the wild type or between the designated strains using the Mann-Whitney U test (**, P < 0.01).
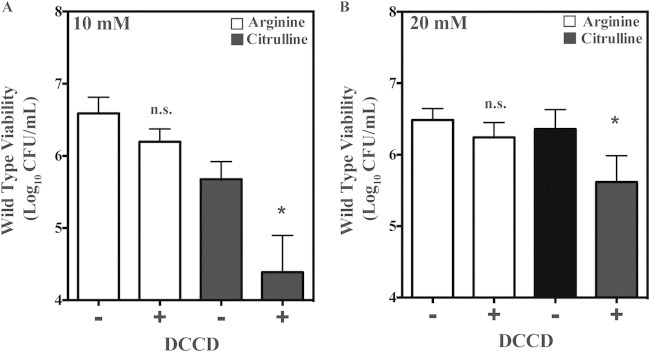
WT protection by citrulline catabolism is sensitive to DCCD.
Understanding the mechanism by which citrulline provides protection against pH stress would provide insight into the important role we observed for citrulline catabolism during infection of soft tissue (5). Comparison of the pH-dependent viability when utilizing citrulline versus arginine revealed that a similar level of bacterial viability was maintained at a slightly lower culture pH when utilizing citrulline (see Fig. S3 in the supplemental material), suggesting that the protection provided relied upon the pH-independent mechanism exhibited by the arcD mutant. To examine the contribution of the F1Fo-ATPase to the mechanism by which citrulline catabolism protects the WT strain, protection was evaluated following treatment with DCCD. To measure DCCD sensitivity, overnight cultures of the WT strain were back-diluted into fresh THY medium supplemented with various concentrations of arginine or citrulline and cultured for an additional 24 h. At this point, DCCD was added to a final concentration of 1.0 mM, incubations were continued for 5 days, and viability was measured by enumeration of CFU. This analysis revealed that with the addition of 10 or 20 mM arginine, there was no significant decrease in CFU between cultures that were challenged with DCCD versus those that were unchallenged (Fig. 8). In contrast, the addition of DCCD resulted in a significant decrease in the mean number of recoverable CFU in the presence of 10 or 20 mM citrulline (Fig. 8). Together, these data demonstrate that while both citrulline and arginine can provide a similar level of protection against acid stress, protection is occurring by different mechanisms, with arginine independent of and citrulline dependent on the F1Fo-ATPase.
FIG 8.
Citrulline protection of the wild type is sensitive to DCCD. Wild-type bacteria were cultured with arginine or citrulline at 10 mM (A) or 20 mM (B) at 37°C for 24 h. Following this growth period, cultures were removed, and either 1 mM DCCD (+) or the vehicle ethanol (−) was added. The cultures were then placed back at 37°C for an additional 5 days. The bacterial viabilities of the cultures were then measured as previously described. Differences in CFU were tested for significance between the designated growth conditions using the Mann-Whitney U test (**, P < 0.05). n.s., not significant.
DISCUSSION
An acidic environment is one of the most common stresses encountered by bacteria both in nature and in infected tissues (1). The present study examined the mechanism by which the ADI pathway of S. pyogenes contributes to adaptation to a low-pH environment. This analysis revealed that ADI-dependent protection consists of two distinct components contributed by catabolism of arginine and citrulline, respectively. The contribution of arginine was associated with its ability to modulate environmental pH, while citrulline's contribution was dependent on the F1Fo-ATPase, implicating a unique role for the citrulline-dependent ATP generated by the ADI pathway in adaptation to acid stress. A distinct role for citrulline in the response to acid stress may explain its contribution to the pathogenesis of S. pyogenes soft tissue infection.
Analysis of the individual ADI pathway mutants allowed the dissection of both the complementary and hierarchical relationships of the protection provided by arginine and citrulline. The complementary nature of protection was revealed by comparison of the ΔArcD and ΔArcC mutants. The arcD mutant was unable to modulate environmental pH (Fig. 4B) and could not generate a conditioned medium that could protect a pH-sensitive mutant (Fig. 6). However, it retained the capacity to protect itself through its ability to produce ATP that could be used by the F1Fo-ATPase to extrude protons to deacidify its cytoplasmic compartment. While the ΔArcD mutant has the ability to produce intracellular ammonia, its sensitivity to acid stress in the presence of an ATPase inhibitor (Fig. 7) indicates that ATP production is more important for protection under these conditions.
In contrast to the ΔArcD mutant, the ΔArcC mutant is incapable of generating ATP. However, it provided near-wild-type levels of protection (Fig. 4A) through the production of ammonia and the buffering of its external environment (Fig. 4B). Its ability to protect itself was not sensitive to inhibition of the ATPase (Fig. 7). Similarly, the WT strain buffered the external medium (Fig. 4B), its conditioned medium could protect a pH-sensitive mutant against acid stress (Fig. 6), and its protective activity was insensitive to inhibition of the ATPase (Fig. 7). These data indicate that although the WT strain has the ability to protect itself both by the production of ATP and by the production of ammonia, the dominant mechanism of protection involves the generation of ammonia and the buffering of the surrounding environment. However, under conditions where arginine concentrations are limiting or when arginine is not metabolized efficiently so that it cannot buffer the extracellular pH, the production of ATP via the ADI pathway can act in concert with the F1Fo-ATPase to provide protection.
Previous studies have shown that hydrolysis of ATP by the F1Fo-ATPase results in the expulsion of three protons from the cytoplasm (20), allowing the bacterium to maintain a higher intracellular pH at the expense of ATP. This mechanism of pH homeostasis provides an explanation for how the catabolism of citrulline by the ADI pathway can protect against acid stress (1). When supplemented with citrulline, the WT strain and those ADI mutants that could still utilize citrulline (e.g., the ΔArcA mutant) were protected from acid stress, but did not significantly raise their extracellular pH (Fig. 4D). Both this activity and protection from acid stress required the activity of the F1Fo-ATPase, demonstrating that the citrulline-based protection has the ability to utilize the nondominant ATP-generating activity of the ADI pathway to provide acid stress protection (Fig. 7 and 8).
The contribution of the F1Fo-ATPase to acid stress protection has been reported for other bacterial species, including Enterococcus hirae, Streptococcus mutans, Streptococcus salivarius, and Listeria monocytogenes (21–23). Similar to S. pyogenes, L. monocytogenes is a human pathogen whose ability to cause disease is dependent on its ability to adapt to acid stress. However, unlike S. pyogenes, L. monocytogenes does not encounter a low-pH environment that results from its own carbon metabolism. Instead, it is an intracellular pathogen that is internalized by a host cell and then must withstand the acidic environment of a phagolysosome (24). In addition to the F1Fo-ATPase, the survival of L. monocytogenes within this intracellular environment also relies upon the ADI pathway (25). In contrast, S. mutans is an extracellular pathogen that is an important agent of dental caries (26). Its pathogenesis depends both on its ability to produce acid to cause the demineralization of dentin at the tooth surface and to its ability to adapt to the extreme-low-pH environment that its metabolism of carbohydrates generates (27). Whether the ADI-mediated catabolism of citrulline can also contribute to the pathogenesis of diseases caused by L. monocytogenes, S. mutans, or other acid-adapting pathogens remains to be determined.
Citrulline is an important metabolic intermediate in the urea cycle and is ubiquitously found in all tissues (28). It can contribute to innate immune defenses against many pathogens, as it can be recycled to arginine by the host enzymes arginosuccinate synthase and arginosuccinate lyase (12, 13). This can produce a steady supply of arginine for the enzyme inducible nitric oxide synthase (iNOS), an important component of the innate immune system that generates nitric oxide, a potent antimicrobial agent (29). We have previously shown that S. pyogenes uses its ADI pathway to modulate innate immunity in the subcutaneous tissues by depleting arginine to impair the iNOS-derived production of nitric oxide (5). This analysis also revealed that those ADI pathway mutants that also could not utilize citrulline were hyperattenuated to a level greater than could be attributed solely to the impairment of iNOS activity (5). The molecular basis underlying hyperattenuation was not understood.
Global analyses of S. pyogenes transcriptional profiles during infection in murine and zebrafish models have indicated that the bacterium is adapting to a low-pH environment (11, 30). Thus, we hypothesized that the hyperattenuation resulting from the loss of citrulline was associated with its arginine-independent ability to aid in the production of ATP for protection against acid stress. However, given that the ability to utilize both arginine and citrulline resides within the ADI pathway, the specific contributions of either substrate to any given phenotype can be difficult to separate. In addition, it has been demonstrated that certain ADI pathway genes may impact pathogenesis via mechanisms independent of their canonical function in catabolism (31). In the present study, we used a comprehensive panel of ADI pathway mutants to systematically evaluate the individual contributions of arginine and citrulline to acid stress adaptation. This analysis has revealed the specific contribution that citrulline can make to protection from acid stress. The application of this approach to other pathogens may prove equally valuable for the analysis of the contributions of arginine and citrulline to pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants NIH 5R01 AI064721 and 5R01 AI070759 from the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02517-14.
REFERENCES
- 1.Cotter PD, Hill C. 2003. Surviving the acid test: responses of Gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453. doi: 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelal AT. 1979. Arginine catabolism by microorganisms. Annu Rev Microbiol 33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- 3.Casiano-Colon A, Marquis RE. 1988. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol 54:1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunin R, Glansdorff N, Pierard A, Stalon V. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev 50:314–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusumano ZT, Watson ME Jr, Caparon MG. 2014. Streptococcus pyogenes arginine and citrulline catabolism promotes infection and modulates innate immunity. Infect Immun 82:233–242. doi: 10.1128/IAI.00916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511. doi: 10.1128/CMR.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barcelona-Andres B, Marina A, Rubio V. 2002. Gene structure, organization, expression, and potential regulatory mechanisms of arginine catabolism in Enterococcus faecalis. J Bacteriol 184:6289–6300. doi: 10.1128/JB.184.22.6289-6300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y, Chen YY, Snyder JA, Burne RA. 2002. Isolation and molecular analysis of the gene cluster for the arginine deiminase system from Streptococcus gordonii DL1. Appl Environ Microbiol 68:5549–5553. doi: 10.1128/AEM.68.11.5549-5553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuniga M, Champomier-Verges M, Zagorec M, Perez-Martinez G. 1998. Structural and functional analysis of the gene cluster encoding the enzymes of the arginine deiminase pathway of Lactobacillus sake. J Bacteriol 180:4154–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelsson L. 1998. Lactic acid bacteria: classification and physiology, p 1–72. In Salminen S, von Wright A (ed), Lactic acid bacteria. Microbiology and functional aspects. Marcel Dekker, Inc, New York, NY. [Google Scholar]
- 11.Loughman JA, Caparon M. 2006. Regulation of SpeB in Streptococcus pyogenes by pH and NaCl: a model for in vivo gene expression. J Bacteriol 188:399–408. doi: 10.1128/JB.188.2.399-408.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori M. 2007. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J Nutr 137(Suppl 2):1616S–1620S. [DOI] [PubMed] [Google Scholar]
- 13.Wu G, Morris SM Jr. 1998. Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Port GC, Paluscio E, Caparon MG. 2013. Complete genome sequence of emm type 14 Streptococcus pyogenes strain HSC5. Genome Announc 1(4):e00612–13. doi: 10.1128/genomeA.00612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanski E, Horwitz PA, Caparon MG. 1992. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun 60:5119–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savic DJ, McShan WM. 2012. Long-term survival of Streptococcus pyogenes in rich media is pH-dependent. Microbiology 158:1428–1436. doi: 10.1099/mic.0.054478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degnan BA, Fontaine MC, Doebereiner AH, Lee JJ, Mastroeni P, Dougan G, Goodacre JA, Kehoe MA. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect Immun 68:2441–2448. doi: 10.1128/IAI.68.5.2441-2448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender GR, Sutton SV, Marquis RE. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun 53:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta AR, Benjamin MM. 1997. Factors controlling acid tolerance of Listeria monocytogenes: effects of nisin and other ionophores. Appl Environ Microbiol 63:4123–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillingame RH, Divall S. 1999. Proton ATPases in bacteria: comparison to Escherichia coli F1F0 as the prototype. Novartis Found Symp 221:218–234. [DOI] [PubMed] [Google Scholar]
- 21.Smith JL, Liu Y, Paoli GC. 2013. How does Listeria monocytogenes combat acid conditions? Can J Microbiol 59:141–152. doi: 10.1139/cjm-2012-0392. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Suzuki T, Unemoto T. 1986. Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J Biol Chem 261:627–630. [PubMed] [Google Scholar]
- 23.Shibata C, Ehara T, Tomura K, Igarashi K, Kobayashi H. 1992. Gene structure of Enterococcus hirae (Streptococcus faecalis) F1F0-ATPase, which functions as a regulator of cytoplasmic pH. J Bacteriol 174:6117–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan S, Hill C, Gahan CG. 2008. Acid stress responses in Listeria monocytogenes. Adv Appl Microbiol 65:67–91. doi: 10.1016/S0065-2164(08)00603-5. [DOI] [PubMed] [Google Scholar]
- 25.Ryan S, Begley M, Gahan CG, Hill C. 2009. Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ Microbiol 11:432–445. doi: 10.1111/j.1462-2920.2008.01782.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsui R, Cvitkovitch D. 2010. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol 5:403–417. doi: 10.2217/fmb.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EG, Spatafora GA. 2012. Gene regulation in S. mutans: complex control in a complex environment. J Dent Res 91:133–141. doi: 10.1177/0022034511415415. [DOI] [PubMed] [Google Scholar]
- 28.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L. 2005. Almost all about citrulline in mammals. Amino Acids 29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 29.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS. 1991. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 30.Neely MN, Pfeifer JD, Caparon M. 2002. Streptococcus-zebrafish model of bacterial pathogenesis. Infect Immun 70:3904–3914. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie H, Lin X, Wang BY, Wu J, Lamont RJ. 2007. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology 153:3228–3234. doi: 10.1099/mic.0.2007/009050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.