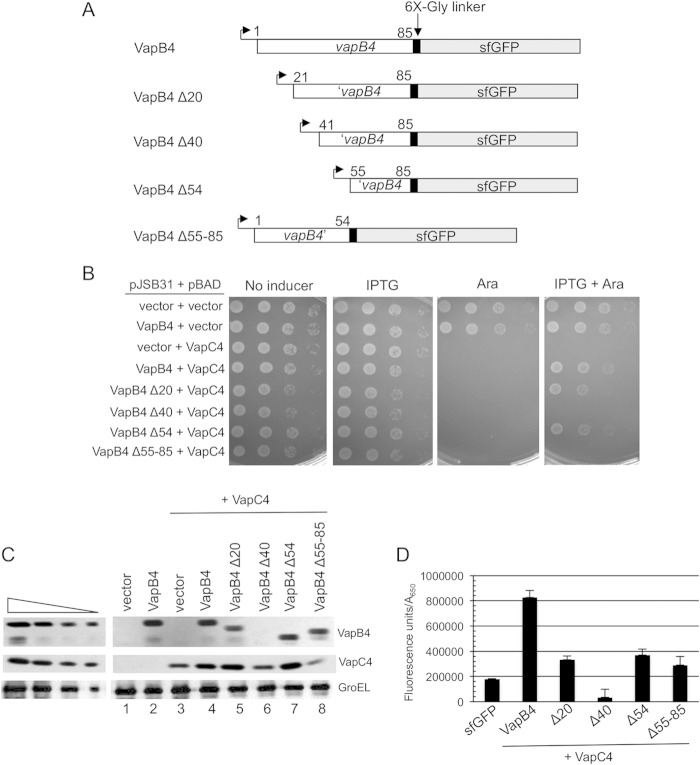
FIG 2.
The C-terminal region of VapB4 (positions 55 to 85) is sufficient for VapB4 antitoxin activity. (A) The open reading frames of VapB4 and its deletion mutants were cloned into the pJSB31-sfGFP plasmid, creating sfGFP fusions. (B) Spotting assay for VapB4 deletion mutants carried out as described in the legend to Fig. 1B. (C) Western blot analysis of cells carrying pJSB31-sfGFP (vector) or pJSB31 carrying the indicated VapB4 alleles and pBAD-VapC4. The cells were grown in M9-glycerol (0.2%) medium at 37°C and induced with 0.02% l-arabinose and 500 μM IPTG for 30 min. Blots were probed with anti-GFP antibody for VapB4 or its mutant proteins and anti-myc antibody for VapC4-myc proteins. Anti-GroEL served as a loading control. The four lanes in each of the panels on the left show a 2-fold dilution series of VapB4-sfGFP, which served as a control for signal linearity. (D) E. coli strain LMG194 carrying the indicated plasmids was grown in M9-glycerol (0.2%) medium supplemented with 50 μg/ml ampicillin and 30 μg/ml chloramphenicol at 37°C to an A600 of 0.1 to 0.2 and induced with 0.02% l-arabinose and 500 μM IPTG for 2 h. GFP fluorescence was measured in a Typhoon 9410 imager and normalized to the OD600 of the culture. Error bars illustrate the standard deviations of triplicate measurements.