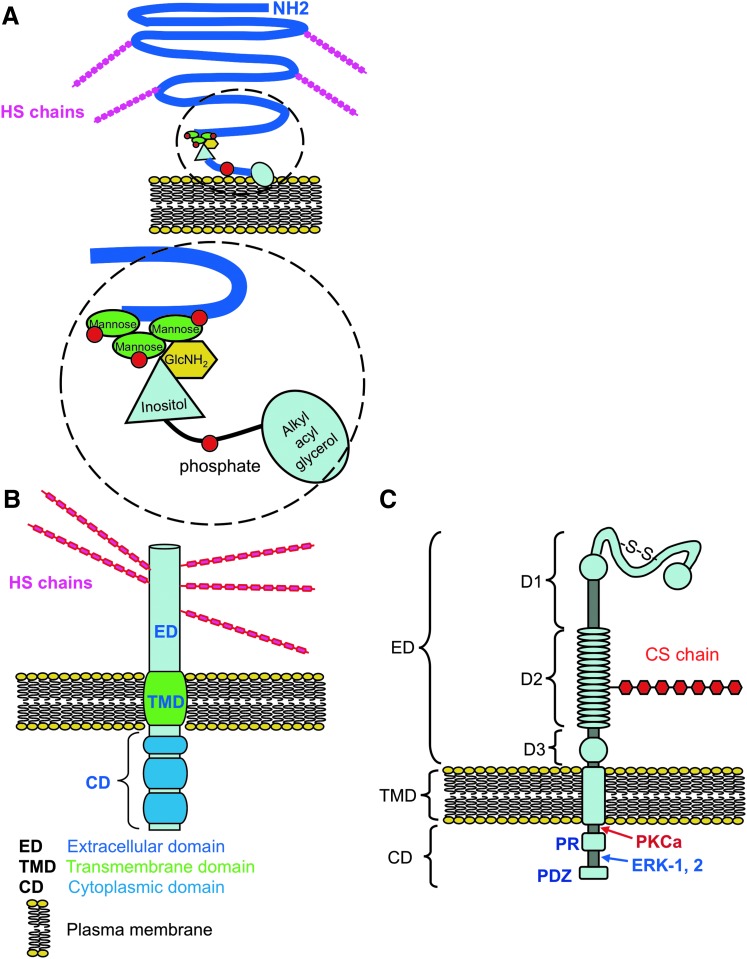
Figure 5.
Structural organization of cell surface proteoglycans identified in skin, glypican (GPC) (A), syndecan (SDC) (B), and nerve-glial antigen-2 (NG2)/chondroitin sulfate proteoglycan (CSPG)-4 (C). Core proteins are depicted in blue, with extracellular (ED), transmembrane (TMD), and cytoplasmic (CD) domains indicated. CS side chains are depicted in NG2 in red and HS chains in SDC and GPC in cerise; the lipid bilayer of the plasma membrane is depicted in yellow. Organization of the extracellular sub domains D1, D2, and D3 of NG2 proteoglycan are also indicated; D1 contains two laminin G type domains, D2 contains a single CS side chain. Although not all forms of NG2 are glycanated, this region of NG2 interacts with type V, VI collagen, growth factors, integrins, and matrix metalloproteinases (MMPs). Two MMP cleavage sites have been demonstrated in the D3 domain, which result in shedding of the extracellular portion of NG2. Protein kinase C alpha (PKCα) and extracellular regulated kinase (ERK)-1, -2 interactive regions in the CD where tyrosine phosphorylation occurs during cell signaling are indicated. GPC contains multiple HS chains in the ED. Detail of the GPC core protein attachment region to the phospholipid bilayer of the plasma membrane by a triphospho-mannose-glucosamine-inositol-glycerophosphate linkage are indicated in the exploded region in (C). Modified from O'Connell and Weeraratna222 and Price et al.84 with permission. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound