Abstract
Introduction: Intrarenal tumors pose a unique challenge to surgeons due to the lack of visual cues on the kidney surface. Intraoperative ultrasonography has facilitated the management of these tumors during minimally invasive partial nephrectomy. We sought to evaluate the safety, feasibility, and comparative effectiveness of robot-assisted partial nephrectomy (RPN) in the management of completely intrarenal tumors.
Methods: Our institutional database was queried for patients undergoing RPN between 2007 and 2013. Patient demographics, tumor characteristics, and perioperative outcomes were compared for patients with intrarenal tumors and tumors with any exophytic component. Patients without available preoperative imaging were excluded from the study.
Results: A total of 297 patients were identified with 30 having completely intrarenal tumors and 267 having some exophytic component. Patient demographics were similar between the two groups. Median tumor size was smaller for the intrarenal group than the exophytic group (2.3 vs 2.7 cm, p=0.015) and nephrometry score was higher for the intrarenal group (9 vs 6, p<0.0001). Tumor characteristics were otherwise similar. Perioperative outcomes were similar between the intrarenal and exophytic groups: estimated blood loss (100 vs 100 mL, p=0.56), operative time (165 vs 162 minutes, p=0.86), warm ischemia time (17 vs 17 minutes, p=0.54), renal cell carcinoma positive surgical margin (0% vs 2.4%, p=0.74), intraoperative complications (0% vs 0.76%, p=0.81), and postoperative complications (6.7% vs 17.6% p=0.76).
Conclusions: RPN is feasible, safe, and effective in the treatment of select intrarenal kidney tumors with outcomes similar to those of partially exophytic tumors. This is likely facilitated by intraoperative ultrasonography. Completely intrarenal kidney tumors should not be automatically relegated to radical nephrectomy or open surgery.
Introduction
Nephron-sparing surgery has become the standard of care for most small renal masses due in part to the reduced associated perceived morbidity when compared to radical nephrectomy (RN).1,2 Completely intrarenal tumors pose a unique technical challenge for surgeons and often RN or open surgery is recommended in place of minimally invasive nephron-sparing surgery for these patients.
Previous reports show that perioperative outcomes for laparoscopic partial nephrectomies (LPNs) are similar between completely intrarenal tumors and tumors with an exophytic component.3,4 The steep learning curve for LPN however restricts its use to highly trained surgeons in order to meet the demands of constraints such as warm ischemia time (WIT).5 Robotic partial nephrectomy (RPN) has been shown to have a shorter learning curve as well as similar or better perioperative outcomes to LPN6–9 and presents a reasonable alternative to LPN in centers with access to robotic surgery. Further, drop-in ultrasound technology allows the surgeon full control of intraoperative imaging. In this study we evaluate the safety, feasibility, and efficacy of RPN for completely intrarenal tumors at a tertiary-care center.
Methods
The institutional-review-board-approved renal mass database at Johns Hopkins was retrospectively queried for consecutive patients who underwent RPN. The tumors were scored using the R.E.N.A.L. nephrometry scoring system.10 The R.E.N.A.L. nephrometry score assigns a complexity to tumors based on (R)adius (maximal tumor diameter), (E)ndophytic/exophytic properties, (N)earness of tumor to the collecting system or sinus, (A)nterior/posterior, (L)ocation relative to polar lines, and the suffix h (hilar) to indicate renal vein or artery involvement. Intrarenal tumors were defined as tumors with no exophytic component and scored with an endophytic/exophytic nephrometry score of 3 (Fig. 1).
FIG. 1.
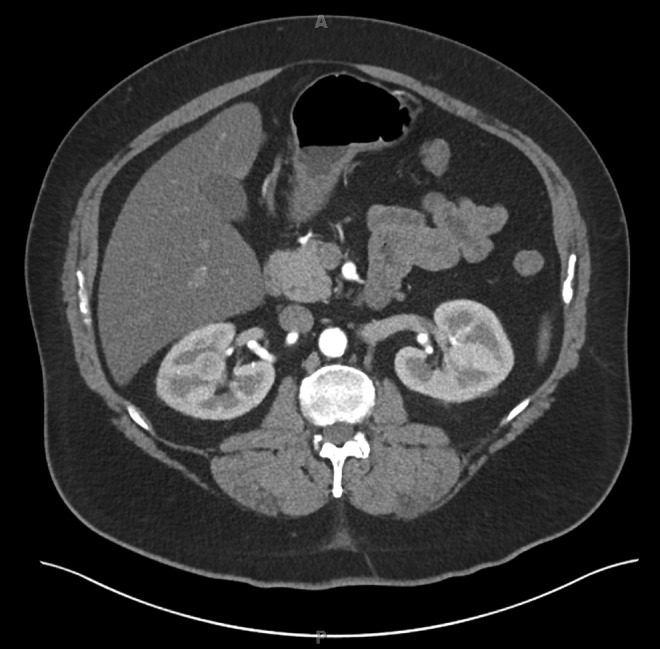
IV-contrast-enhanced CT showing a completely intrarenal tumor in the right kidney.
A total of 297 consecutive patients undergoing RPN from 2007 to 2013 with preoperative imaging (CT or MRI) were identified. Patients without available preoperative imaging were excluded from analysis.
Statistical analyses were performed to compare completely intrarenal tumors with the remainder of the cohort. Preoperative patient and tumor characteristics and perioperative outcomes, including WIT, estimated blood loss (EBL), total operative time, positive margin rate, and complication rate,11 were compared between groups. The Mann–Whitney U test was used for continuous variables and Fisher's exact for categorical variables. Statistical significance was considered to be p<0.05. Statistical calculations were made using Stata 13 (StataCorp LP, College Station, TX).
Surgical technique
All patients underwent RPN via a transperitoneal approach, using a 3- or 4-arm robotic technique. After lifting the kidney off of the psoas muscle, the renal hilum was approached to identify the renal artery and vein. Gerota's fascia was entered and the kidney was defatted widely over the general area of tumor localization on preoperative imaging. A laparoscopic or robotic drop-in ultrasound was utilized to identify the tumor. Correlating the ultrasound image with the preoperative cross-sectional images, the surgeon scored the capsule to define the borders of resection. Hilar control was then obtained and tumor excision was then carried out with cold scissors. For most tumors, sinus fat was used to determine the depth of the resection and ensure a clear margin. Collecting system entries and large vessels were closed with running 3-0 polyglactin sutures following tumor resection. The renal parenchyma was approximated with 2-0 polyglactin suture utilizing the sliding-clip technique.12 A retroperitoneal drain was left in place in all cases. The tumor was placed into an extraction bag and in all cases the specimen was bivalved following extraction to confirm complete tumor excision.
Results
Of the 297 patients in our cohort, 30 (10.1%) were identified as having completely intrarenal tumors. The patient characteristics are listed in Table 1. There was no significant difference in age, body mass index, American Society of Anesthesiologists score, Charlson comorbidity index, or gender between the two groups. Tumor characteristics are also listed in Table 1. Tumor size was smaller in the intrarenal group (2.3 vs 2.7 cm, p=0.015). Intrarenal tumors had a significantly higher nephrometry score than exophytic tumors (9 for intrarenal vs 6, p<0.0001). There was no significant difference between pathologic classification and T stage between the two groups. The most common renal cell carcinoma (RCC) subtype was clear cell and most common pathologic stage was pT1a.
Table 1.
Clinicopathologic Characteristics of Patients with Completely Intrarenal Tumors Compared with Tumors with an Exophytic Component
| Completely intrarenal tumors (n=30) | Exophytic component to tumor (n=267) | p-Value | |
|---|---|---|---|
| Age | 54.5 (49.8–63.3) | 60.9 (53.1–67.1) | 0.11 |
| BMI | 30.2 (25.5–34.5) | 29.4 (25.3–33.2) | 0.37 |
| Male, n (%) | 14 (46.7) | 170 (63.4) | 0.078 |
| ASA | 2 (2–3) | 2 (2–3) | 0.69 |
| CCI | 0 (0–2) | 0 (0–1) | 0.72 |
| Tumor diameter (cm) | 2.3 (1.4–2.9) | 2.7 (1.9–3.8) | 0.015 |
| Nephrometry score | 9 (8–10) | 6 (5–8) | <0.0001 |
| RCC, n (%) | 20 (66.7) | 208 (77.6) | 0.18 |
| RCC subtype, n (%) | 0.55 | ||
| Clear cell | 15 (75.0) | 110 (52.9) | |
| Papillary | 2 (10.0) | 45 (21.6) | |
| Chromophobe | 0 (0) | 20 (9.6) | |
| Other | 3 (15.0) | 33 (15.9) | |
| Pathologic T stage, n (%) | 0.26 | ||
| pT1a | 19 (95.0) | 161 (77.4) | |
| PT1b | 0 (0) | 31 (14.9) | |
| PT2a | 0 (0) | 3 (1.4) | |
| PT3a | 1 (5.0) | 13 (6.3) |
Values are expressed as medians±interquartile range unless otherwise specified.
ASA=American Society of Anesthesiologists; BMI=body mass index; CCI=Charlson comorbidity index; RCC=renal cell carcinoma.
Operative parameters and perioperative outcomes are shown in Table 2. There was no significant difference in EBL, operative time, WIT, positive margin rate, or complication rate between intrarenal tumors and exophytic tumors. In the exophytic group, there were 22 operations that used a zero ischemia approach; these operations were excluded for the purposes of calculating WIT. There were no recurrences in either group with a median follow-up of 10.6 months.
Table 2.
Perioperative Outcomes of Patients with Completely Intrarenal Tumors Compared with Tumors with an Exophytic Component
| Completely intrarenal tumors (n=30) | Exophytic component to tumor (n=267) | p-Value | |
|---|---|---|---|
| EBL (mL) | 100 (100–150) | 100 (50–150) | 0.56 |
| Operative time (minutes) | 165 (139–189) | 162 (141–200) | 0.86 |
| WIT (minutes) | 17 (14–20) | 17 (13–20) | 0.54 |
| RCC positive surgical margin, n (%) | 0 (0) | 5 (2.4) | 0.74 |
| Intraop complications, n (%) | 0 (0) | 2 (0.76) | 0.81 |
| Postop complications (%) | 0.76 | ||
| Overall | 2 (6.7) | 47 (17.6) | |
| Clavien 1–2 | 2 (6.7) | 38 (14.2) | |
| Clavien 3–4 | 0 (0) | 9 (3.4) |
Values are expressed as medians±interquartile range unless otherwise specified.
EBL=estimated blood loss; WIT=warm ischemia time.
Discussion
The gold-standard treatment for small renal masses is PN.13,14 Hollenbeck et al., however, report that partial nephrectomies (PNs) are underutilized and more likely to be performed at teaching institutions with high volumes.15 Intrarenal tumors present an additional challenge in that they are completely enclosed by renal parenchyma and there are often no visual cues when resecting the tumor. Intraoperative ultrasound is required to locate and excise the tumor. Given these challenges, it is not surprising that RN or open surgery remains a popular management option for these tumors.
When nephron-sparing surgery is desired, open approaches have historically been used. As LPN emerged as a surgical technique, it became a new standard of care.16 Minimally invasive approaches, such as LPN, have been shown to have decreased operative time, blood loss, and length of stay while maintaining similar oncologic outcomes when compared with open PNs.17 Previous studies have shown LPN as a safe and effective approach for treating completely intrarenal tumors3,4; however, these results were in the hands of experienced laparoscopic surgeons and similar results have not been reported outside these centers of excellence. The technical challenges and learning curve for LPN may keep the surgery out of the reach of many surgeons and patients.18
Recently, there has been a dramatic rise in the number of RPNs performed. In just a few years, RPN has overtaken LPN as the approach of choice for minimally invasive procedures for PN.19 Patel et al. showed in a recent study that the use of PNs is on the rise, and this increase is being facilitated by robotic technology.20 RPN may also enable more complex tumor resection to be performed without conversion to RN.21
Our study presents a single-institution experience with RPN for intrarenal tumors. Compared with previous LPN series,3,4 our results compare favorably. In our analysis RPN for intrarenal lesions behaved similarly to the exophytic cohort in terms of EBL, operative time, WIT, positive surgical margins, and complications. These results are promising and offer a minimally invasive nephron-sparing approach to intrarenal tumors at centers that do not have the expertise to perform LPN but have access to and experience with robotic surgery.
In their recent retrospective study, Autorino et al. showed similar results to ours.22 In contrast to our results, they found an increased WIT when treating intrarenal tumors. Even with this increase, their mean WIT of 21.7 minutes for intrarenal tumors is within the range of time normally seen in RPN. These results together with ours show that RPN is a safe and effective treatment for intrarenal tumors and offers a reasonable alternative to LPN for these tumors.
Several considerations are important when tackling these tumors. Urothelial carcinoma should be considered if the lesion is infiltrative or atypical in appearance. If suspected, then this possibility should be excluded via further testing (ureteroscopy±urine cytology). We routinely obtain a preoperative ultrasound in order to optimally counsel patients especially those harboring the rare tumor that is isoechoic and difficult to distinguish from adjacent normal kidney. These patients need to be prepared for difficulty (or inability) in localizing their tumor intraoperatively. Additionally, patients should be counseled that higher-complexity tumors have an increased risk of upstaging to pathologic T3a disease on final pathology.23 Less-experienced surgeons should gain comfort and familiarity with RPN before tackling these more technically demanding tumors so that a safe and oncologically efficacious is performed. Finally, active surveillance is always considered a management alternative for these small renal tumors.
The major limitations of this study are the relatively small number of intrarenal tumors and the retrospective, single-institution study design. The small sample size may limit the power to detect differences in outcome measures between the two study groups. Further, the majority of cases were performed by surgeons experienced in both LPN and RPN at high-volume tertiary cancer center. The results may not be generalizable to other institutions and to surgeons early in their learning curve. Finally, there is likely a selection bias toward intrarenal tumors more amenable to partial nephrectomy.
Conclusions
RPN for completely intrarenal tumors is safe and feasible, and represents an alternative to open surgery, RN, and LPN for these tumors. Our study shows no significant difference in perioperative outcomes when compared with tumors with an exophytic component. As robotic technology continues to be more widespread, patients who historically would require an RN will hopefully be able to benefit from the advantages of nephron-sparing surgery.
Abbreviations Used
- RPN
robotic partial nephrectomy
- RN
radical nephrectomy
- LPN
laparoscopic partial nephrectomy
- CT
computed tomography
- MRI
magnetic resonance imaging
- EBL
estimated blood loss
- WIT
warm ischemia time
- RCC
renal cell carcinoma
- BMI
body mass index
- ASA
American Society of Anesthesiologists
- CCI
Charlson comorbidity index
Disclosure Statement
No competing financial interests exist.
References
- 1.Touijer K, Jacqmin D, Kavoussi LR, et al. . The expanding role of partial nephrectomy: A critical analysis of indications, results, and complications. Eur Urol 2010;57:214–222 [DOI] [PubMed] [Google Scholar]
- 2.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009;181:55–61; discussion 61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung BI, Lee UJ, Kamoi K, Canes DA, Aron M, Gill IS. Laparoscopic partial nephrectomy for completely intraparenchymal tumors. J Urol 2011;186:2182–2187 [DOI] [PubMed] [Google Scholar]
- 4.Nadu A, Goldberg H, Lubin M, Baniel J. Laparoscopic partial nephrectomy (LPN) for totally intrarenal tumours. BJU Int 2013;112:E82–E86 [DOI] [PubMed] [Google Scholar]
- 5.Patel AR, Eggener SE. Warm ischemia less than 30 minutes is not necessarily safe during partial nephrectomy: Every minute matters. Urol Oncol 2011;29:826–828 [DOI] [PubMed] [Google Scholar]
- 6.Benway BM, Bhayani SB, Rogers CG, et al. . Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: A multi-institutional analysis of perioperative outcomes. J Urol 2009;182:866–872 [DOI] [PubMed] [Google Scholar]
- 7.DeLong JM, Shapiro O, Moinzadeh A. Comparison of laparoscopic versus robotic assisted partial nephrectomy: One surgeon's initial experience. Can J Urol 2010;17:5207–5212 Available at: www.ncbi.nlm.nih.gov/pubmed/20566016 Accessed February23, 2014 [PubMed]
- 8.Mullins JK, Feng T, Pierorazio PM, Patel HD, Hyams ES, Allaf ME. Comparative analysis of minimally invasive partial nephrectomy techniques in the treatment of localized renal tumors. Urology 2012;80:316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierorazio PM, Patel HD, Feng T, Yohannan J, Hyams ES, Allaf ME. Robotic-assisted versus traditional laparoscopic partial nephrectomy: Comparison of outcomes and evaluation of learning curve. Urology 2011;78:813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844–853 [DOI] [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 Available at: www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1360123&tool=pmcentrez&rendertype=abstract Accessed February22, 2014 [DOI] [PMC free article] [PubMed]
- 12.Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: Technique and outcomes. Eur Urol 2009;55:592–599 [DOI] [PubMed] [Google Scholar]
- 13.Guidelines for Management of the Clinical Stage 1 Renal Mass. Available at: www.auanet.org/education/guidelines/renal-mass.cfm Accessed February10, 2014
- 14.Ljungberg B, Cowan NC, Hanbury DC, et al. . EAU guidelines on renal cell carcinoma: The 2010 update. Eur Urol 2010;58:398–406 [DOI] [PubMed] [Google Scholar]
- 15.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: A case of underutilization? Urology 2006;67:254–259 [DOI] [PubMed] [Google Scholar]
- 16.Makhoul B, De La Taille A, Vordos D, et al. . Laparoscopic radical nephrectomy for T1 renal cancer: The gold standard? A comparison of laparoscopic vs open nephrectomy. BJU Int 2004;93:67–70 Available at: www.ncbi.nlm.nih.gov/pubmed/14678371 Accessed March2, 2014 [DOI] [PubMed]
- 17.Gill IS, Kavoussi LR, Lane BR, et al. . Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 2007;178:41–46 [DOI] [PubMed] [Google Scholar]
- 18.Porpiglia F, Bertolo R, Amparore D, Fiori C. Margins, ischaemia and complications rate after laparoscopic partial nephrectomy: Impact of learning curve and tumour anatomical characteristics. BJU Int 2013;112:1125–1132 [DOI] [PubMed] [Google Scholar]
- 19.Ghani KR, Sukumar S, Sammon JD, Rogers CG, Trinh Q-D, Menon M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: Results from the nationwide inpatient sample. J Urol 2014;191:907–912 [DOI] [PubMed] [Google Scholar]
- 20.Patel HD, Mullins JK, Pierorazio PM, et al. . Trends in renal surgery: Robotic technology is associated with increased use of partial nephrectomy. J Urol 2013;189:1229–1235 [DOI] [PubMed] [Google Scholar]
- 21.Long J-A, Yakoubi R, Lee B, et al. . Robotic versus laparoscopic partial nephrectomy for complex tumors: Comparison of perioperative outcomes. Eur Urol 2012;61:1257–1262 [DOI] [PubMed] [Google Scholar]
- 22.Autorino R, Khalifeh A, Laydner H, et al. . Robotic partial nephrectomy for completely endophytic renal masses: A single institution experience. BJU Int 2014;113:762–768 [DOI] [PubMed] [Google Scholar]
- 23.Gorin MA, Ball MW, Pierorazio PM, et al. . Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: A multi-institutional analysis. J Urol 2013;190:1907–1911 [DOI] [PubMed] [Google Scholar]


