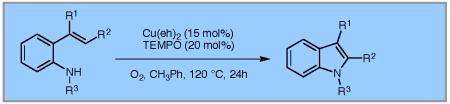
Table 2.
Substrate Scopea
| |||
|---|---|---|---|
|
| |||
| entry | substrate | product | yieldb |
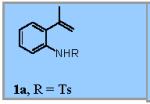
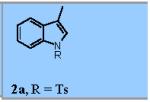
| 1 |
|
|
71% |
| 2 | 1b, R = SES | 2b, R = SES | 55% |
| 3 | 1c, R = Ns | 2c, R = Ns | 51% |
| 4 | 1d, R = Ms | 2d, R = Ms | 50% |
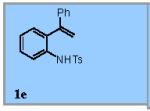
| 5 |
|
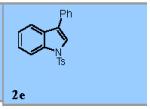
|
73% |
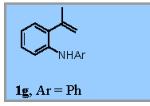
| 6 |
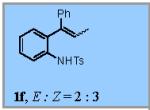
|
|
22% |
| 7 |
|
|
43% |
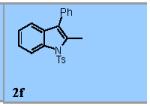
| 8 |
|
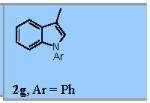
|
83% |
| 8 | 1h, Ar = 4-F-Ph | 2h, Ar = 4-F-Ph | 81% |
| 9 | 1i, Ar = 4-MeO-Ph | 2i, Ar = 4-MeO-Ph | 85% |
| 10 |
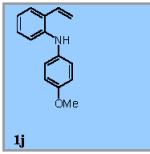
|
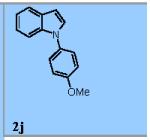
|
55% |
Reaction conditions used: 1a (0.174 mmol, 1 equiv), Cu(eh)2 (0.026 mmol, 15 mol %), TEMPO (0.035 mmol, 20 mol %), toluene (1.74 mL) and O2 (1 atm, balloon).
Isolated yield after column chromatography
22% of the (Z)-isomer of 1f was recovered.
SES = 2-(trimethylsilyl)ethanesulfonyl, Ns = 4-nitrophenylsulfonyl, Ms = methane sulfonyl.
