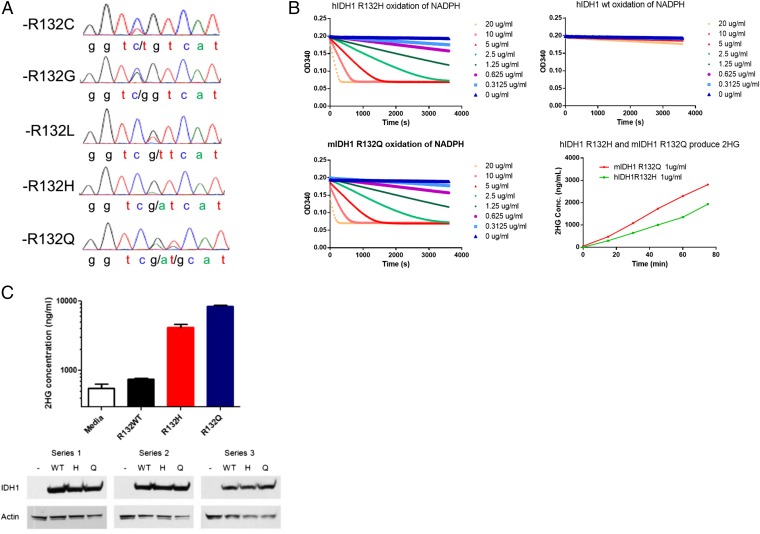
Fig. 1.
IDH1 R132Q mutations confer an enzymatic activity that converts α-KG to d-2HG. (A) Chromatograms generated by Sanger sequencing at the locus coding IDH1-R132 in human chondrosarcoma samples. (B) NADPH consumption and d-2HG production by IDH1 mutant proteins. Recombinant R132Q, R132H, and WT IDH1 proteins were assayed for NADPH consumption activity at different protein concentrations. This neoactivity is acquired by known IDH1 driver mutations. MS identified the production of d-2HG by R132Q and R132H mutant proteins. Liquid chromatography (LC) /MS was used to identify and measure the concentration of d-2HG in enzymatic assays of recombinant protein by comparing spectra with a d-2HG standard. Time-dependent d-2HG production was detected in the reactions catalyzed by IDH1 R132Q and IDH1 R132Q proteins but not WT protein. (C) Cellular production of d-2HG by R132Q and R132H mutant IDH1. HEK293 cells were transiently transfected with expression plasmids containing WT IDH1 or R132H or R132Q mutations. After 48 h, d-2HG concentration in the culture media was measured by LC/MS and compared with a media alone control, and protein expression was assessed by Western blot.