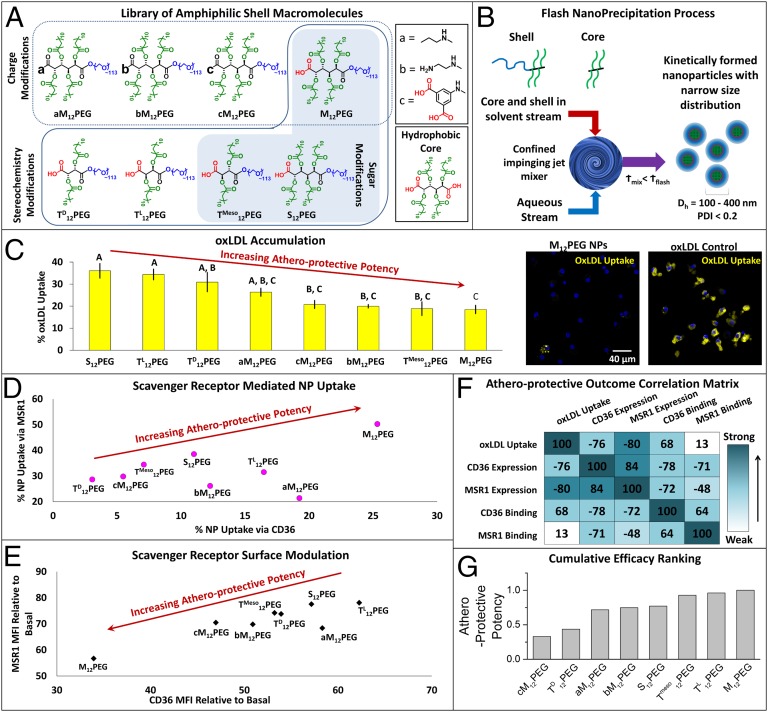
Fig. 2.
AM NPs were designed, fabricated, and screened for multiple measures of bioactivity, and the most atheroprotective NP formulation was identified. (A) A library of AMs, with changes in charge, stereochemistry, and sugar backbones, was designed and synthesized, and (B) kinetically fabricated into serum-stable, core/shell nanoparticles via flash nanoprecipitation. In vitro screening of NP bioactivity was measured via several atherogenic endpoints in hMDMs: (C) inhibition of oxLDL accumulation, (D) NP binding and uptake via key scavenger receptors MSR1 and CD36, and (E) NP modulation of surface expression of MSR1 and CD36. (F) Correlation analysis found varied relationships between these five measures of atheroprotective outcomes. (G) The biological outcomes were weighted and NPs were cumulatively ranked for efficacy. For C and E, the cells were coincubated with 5 μg/mL oxLDL and 10−5 M NPs for 24 h, whereas for D, the cells were incubated with polyinosinic acid (MSR1 inhibitor) or CD36 blocking antibody (CD36 inhibitor) for 2 h and then incubated with 10−5 M NPs for 6 h, and all analysis was evaluated by flow cytometry. Error bars represent SEM and n = 3. For C, all treatments are P < 0.0001 from oxLDL only control and those with the same letter are statistically indistinguishable. The control nonbioactive nanoparticle (PS14PEG shell, PS14 core) demonstrated no atheroprotective bioactivity (SI Appendix, Fig. S4).