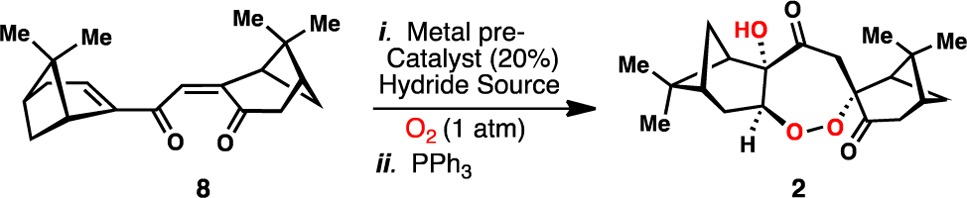
Table 1. Metal-Catalyzed Synthesis of 2: Selected Optimizationa,b.
| entry | conditions | isolated yield (%)a |
|---|---|---|
| 1 | Fe2(ox)3·6H2O (5 equiv), NaBH4 (6.4 equiv), EtOH/H2O, 0 °C | 0 |
| 2 | Fe∥(Pc), NaBH4 (3.0 equiv), EtOH, 0 °C | 0 |
| 3 | Fe(acac)3, PhSiH3 (2.5 equiv), EtOH, 0 °C → rt | 0 |
| 4 | Co(acac)2, PhSiH3 (2.5 equiv), DCM/i-PrOH, –10 °C → rt | 6 |
| 5 | Mn(dpm)3, PhSiH3 (2.5 equiv), DCM/i-PrOH, –10 °C | 34 |
| 6 | Mn(dpm)3, PhSiH3 (2.5 equiv), DCM/i-PrOH, –10 °C | 41b |
| 7 | Mn(dpm)3, PhSiH3 (2.5 equiv), t-BuOOH (1.5 equiv), DCM/i-PrOH, –10 °C | 52b |
Reaction performed on a 0.1 mmol scale using 20 mol % of metal catalyst unless otherwise stated.
Phenylsilane added slowly over 12 h as a solution in DCM. Pc = Phthalocyanine. ox = oxalate, acac = acetylacetonate.
