Scheme 13. Completion of the Synthesis of Ouabagenin (1).
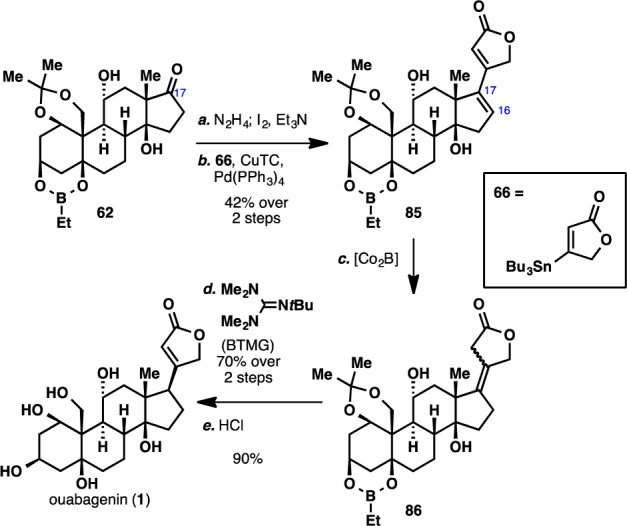
Reagents and conditions: (a) N2H4 (10 equiv), Et3N (10 equiv), 4:1 CH2Cl2:EtOH, 50 °C, 5 h; I2 (3 equiv), Et3N (4 equiv), THF, 10 min; (b) 66 (4 equiv), [Ph2PO2][NBu4] (4 equiv), Pd(PPh3)4 (0.15 equiv), CuTC (3 equiv), DMF, 23 °C, 2 h, 42% over two steps; (c) CoCl2·6H2O (2.5 equiv), NaBH4 (5 equiv), EtOH, 0 to 23 °C, 20 min; (d) BTMG (1.5 equiv), C6H6, 100 °C, 10 min, 70% over two steps; (e) conc. HCl (2 equiv), MeOH, 23 °C, 30 min, 90%.
