Figure 2.
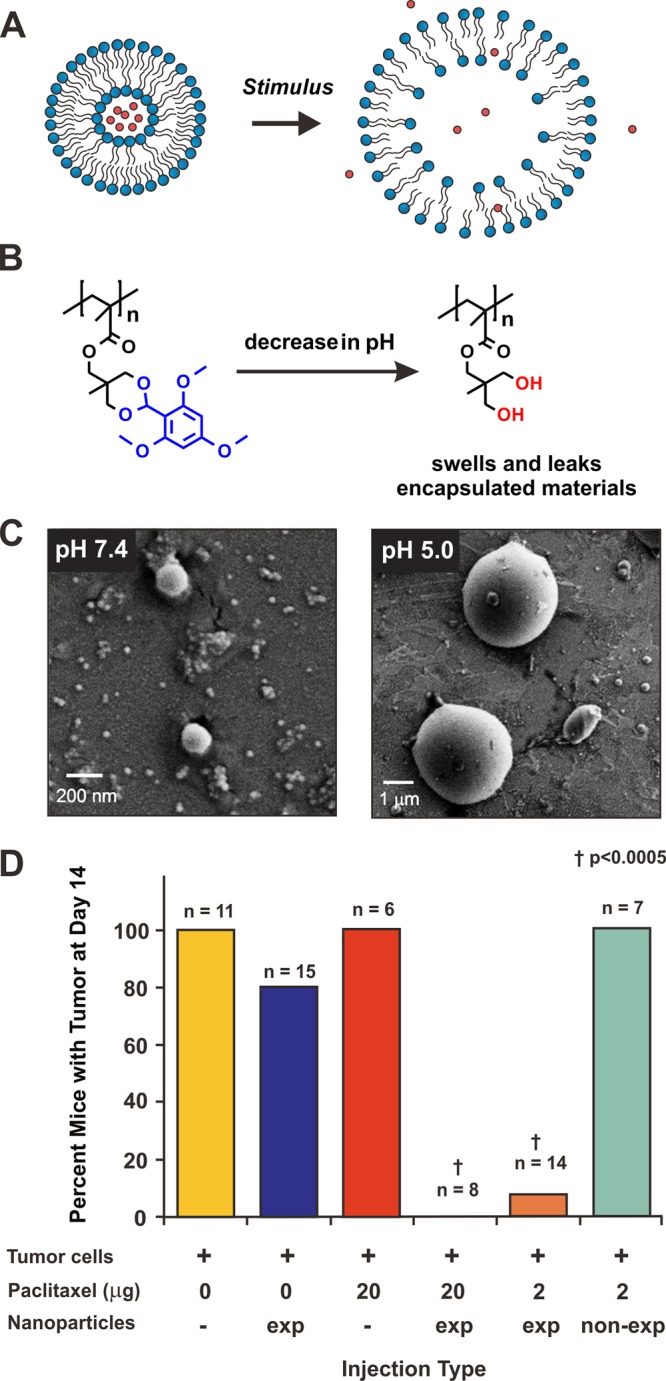
Expansile nanoparticle systems. (A) A general cartoon describing systems that release encapsulated drugs (red circles) by expansion into a fenestrated structure upon activation by a stimulus. (B) Chemical structures of expansile nanoscale particles containing 2,4,6-trimethoxylbenzaldehyde-derived acetals that hydrolyze at pH ≤ 5 to yield diol-containing scaffolds, which form micrometer-scale hydrogels. The pH of activation is in line with the pH of cellular lysosomes, and so these materials have been used to release encapsulated drugs inside cellular organelles upon internalization. (C) Scanning electron microscopy images of the acetal described in B at pH 7.4, and the diol hydrolysis product at pH 5.0. Note that the diameter of the material expands 350-fold in response to mildly acidic environments. (D) Experimental data from in vivo studies in which C57BL/6 mice are injected with Lewis lung carcinoma cells alongside paclitaxel-loaded expansile (exp) and non-expansile particles (non-exp, which contain related benzaldehyde-derived acetals) and appropriate controls. Note that only mice that received the drug-loaded exapansile particles were free from tumors. Panels C and D are adapted from Colby et al.48 with permission from the Royal Society of Chemistry and Griset et al. with permission from the American Chemical Society,142 respectively.
