Figure 7.
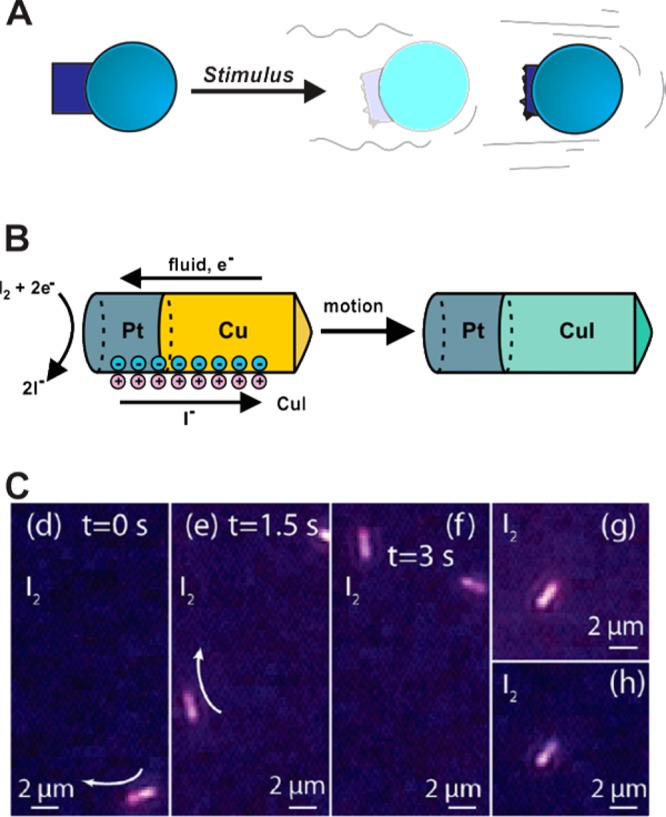
Nanomaterials capable of autonomous motion. These materials are often prepared by incorporating motors that use catalysis to generate chemical concentration gradients and propel themselves through solution via self-electrophoresis. (A) Cartoon depiction of the motion of a nanomaterial generated via the depletion of chemical fuel placed on one side of the object. (B) Platinum–copper nanorods that catalyze the reduction of iodine to iodide while oxidizing copper at the opposite end of the rod. The reduction that takes place at the platinum end of the nanorod generates a flow of electrons toward the platinum end of the material. This electron flow generates a charge differential, thus inducing fluid movement toward the platinum segment and propelling the nanorod in the opposite direction of the fluid. (C) Optical microscopy snapshots tracking movement of Pt–Cu nanorods over time (images (g) and (h) depict instances of surface-immobilized nanorods). Panels B and C are adapted from Liu et al.191 with permission from the American Chemical Society.
