Abstract
Glaucoma, the leading cause globally of irreversible blindness, is a neurodegenerative disease characterized by progressive retinal ganglion cell death. To date, no drug has been shown to prevent the retinal ganglion cell loss associated with glaucoma. Multiple mechanisms lead to ganglion cell death in glaucoma, suggesting that a neuroprotectant that has a single mode of action, like memantine, would have a limited positive effect at slowing down ganglion cell death. Conversely, simultaneously targeting several factors may be the best therapeutic approach to improve outcomes. Multifunctional drugs are fast gaining acceptance as a strategy for the treatment of complex disorders of the central nervous system, such as Parkinson's disease, Alzheimer's disease and other progressive neurodegenerative diseases. In this paper, we review the current literature on multifunctional drugs and propose a rationale for the use of multifunctional drugs in glaucomatous optic neuropathy.
Keywords: multifunctional drugs, glaucoma, neuroprotection, neurodegenerative disease, retinal ganglion cells, reviews
INTRODUCTION
Glaucoma is currently recognized as a multifactorial neurodegenerative disorder with complex pathogenesis, affecting 60 million people worldwide in its most common forms[1,2,3]. Elevated intraocular pressure (IOP) is a major risk factor for glaucoma; however, traditional strategies of lowering intraocular pressure have been shown to be unable to prevent progressive vision loss in some glaucoma patients, so the focus of glaucoma research has shifted toward neuroprotection[2,4]. Neuroprotection for glaucoma can be considered an additional therapeutic strategy, independent of and complementary to IOP-lowering treatment, directly targeting retinal ganglion cells (RGCs) and neurons of the higher visual centers[5,6].
One such strategy is pharmacological neuroprotection, in which a drug is administered to interact with neuronal or glial elements within the retina/optic nerve head and thereby facilitate the survival of RGCs[4]. Two recent parallel clinical trials of oral memantine, an N-methyl-D-aspartate (NMDA) antagonist, in patients with chronic progressive open angle glaucoma, were unsuccessful[7,8]. The results of industry-supported trials have not yet been published, but the sponsor of these trials has provided press releases[8]. Currently, no drug with claimed neuroprotective activity has been identified or approved for the treatment of glaucoma. Multiple mechanisms lead to ganglion cell death in glaucoma, suggesting that a neuroprotectant that has a single mode of action, like memantine, would have a limited positive effect at slowing down ganglion cell death. Conversely, simultaneously targeting several factors may be the most likely therapeutic approach to improve outcome.
In recent years, there has been a rapidly expanding interest in multifunctional drugs as an approach to the treatment of central nervous system neurodegenerative disorders with complex pathological mechanisms[9,10,11,12]. Increasing evidence shows that numerous similarities exist between glaucomatous neurodegeneration and other central nervous system neurodegenerative diseases[13,14,15,16]. Excitotoxicity triggered by elevated glutamate, excess concentrations of amyloid β peptide and excessive oxidant damage by reactive oxygen species have been implicated in the development of neurodegenerative process of glaucoma, as they have in Alzheimer's disease[16,17,18]. Additionally, there is a growing trend toward using existing neuroprotective strategies in central nervous system neurodegenerative diseases for the treatment of glaucoma[2].
RATIONALE FOR DEVELOPING MULTIFUNCTIONAL DRUGS FOR NEUROPROTECTION
The incidence of neurodegenerative diseases has increased steadily worldwide owing to the extended life expectancy brought about by better health care. These neurodegenerative diseases include Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, and multiple system atrophy, as well as diseases originating from an acute initial insult such as traumatic brain injury and stroke. Glaucoma is a neurodegenerative disease, and the incidence of neurodegenerative diseases tends to increase with aging, as is also observed in glaucoma[19]. A considerable body of evidence shows that the pathogenesis of these diseases is extremely complex and heterogeneous, resulting in significant comorbidity and that they are therefore unlikely to be mitigated by any drug acting on a single pathway or target[20,21].
The one-target one-drug paradigm has been the dominating drug discovery approach since the early 1990s. This paradigm attempts to identify a single chemical entity that binds to a single target[22,23]. Drug discovery in neurodegenerative diseases has followed the same trend. For instance, memantine, an NMDA receptor (NMDAR) channel antagonist, was developed for the treatment of moderate-to-severe Alzheimer's disease in 2003[24]. However, owing to disappointing clinical results, the effectiveness of this paradigm has been questioned. Recent large-scale genomics studies have confirmed significant redundancy in proteinaceous drug targets, suggesting that drugs directed toward single pathophysiological mechanisms may have more limitations than multifunctional drugs[11,25].
Multifunctional drugs are those agents with more than one therapeutic mechanism[26]. When an undesired pharmacologic action occurs at therapeutic doses, this is not a multifunctional drug, but a “dirty drug”. More recently, a new paradigm that addresses disease etiological complexity using a multi-target-directed ligand approach has gained increasing acceptance. Novel compounds are specifically designed to target the multiple mechanisms underlying the etiology of a specific disease, and these have shown superior efficacy and safety profiles. These agents offer the promise of preventing, arresting, or slowing decline through disease modification. Thus, the major drug discovery paradigm is shifting from a one-drug–one-target strategy to a one-drug-multiple-targets strategy (Figure 1).
Figure 1.
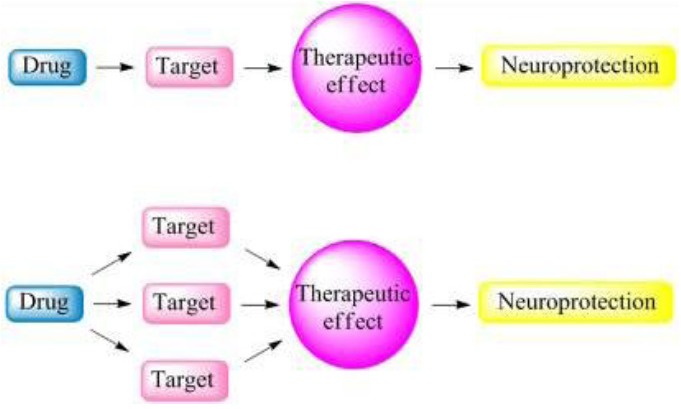
The drug discovery strategy for complex pathologies such as those found in neurodegenerative disease has undergone a paradigm shift from the design of one-molecule-one-target agent to the design of multifunctional drugs.
In 2006, rasagiline (N-propagrgyl-1R-aminoindan) was approved as the first once-daily oral treatment for Parkinson's disease by the U.S. Food and Drug Administration[10]. It was also the first Parkinson's disease treatment to receive the label “disease-modifying”[27]. The development of rasagiline provides an excellent example to support the validity of the multi-target-designed ligand approach to searches for effective medicines combating neurodegenerative diseases.
POTENTIAL MULTIPLE TARGETS FOR NEUROPROTECTION IN GLAUCOMA
Glaucoma is a leading cause of irreversible world vision loss and is characterized by progressive RGC death. Although the exact mechanism underlying glaucoma remains uncertain, much progress has been made in identifying potential pharmacological targets. The development of animal models of chronic glaucoma has enhanced our understanding of many of the pathological processes occurring in glaucoma and, in doing so, has suggested logical targets for pharmacological intervention.
Glutamate is a major excitatory neurotransmitter in the central nervous system, including the retina. Glutamate-mediated excitotoxicity, primarily through NMDARs, may be an important cause of RGC death in glaucoma[2,4,28]. Pharmacological inhibition of NMDARs has been advocated to be an important strategy for neuroprotection in glaucoma. Memantine, an NMDA antagonist, has undergone phase 3 clinical trials for glaucoma progression, but the drug did not show significant efficacy in preserving visual function[6,8].
It has been suggested that excessive nitric oxide is involved in the optic neuropathy associated with glaucoma, and that it is most likely made by reactive astrocytes and microglia in the optic nerve heads[29,30,31]. Nitric oxide is generated by the action of nitric oxide synthase (NOS), which has three different forms, namely, neuronal NOS (nNOS), endothelial and inducible. In a rat model of chronic glaucoma, nNOS expression was significantly increased and a non-specific NOS inhibitor reduced RGC loss[32]. Another investigation showed that inducible nitric oxide synthase did not mediate optic neuropathy and retinopathy in the DBA/2J glaucoma model[33]. Thus, it has been suggested that pharmacological inhibition of nNOS represents a novel strategy for the treatment of glaucoma[32].
It is commonly believed that intracellular calcium is a major mediator of neuronal cell death in ischemia, in which high levels of released glutamate can produce overstimulation of ionotropic glutamate receptors, leading to neuronal cell death triggered by a large influx of Ca2+ into cells mainly via NMDARs and secondary opening of voltage-dependent Ca2+ channels[34,35]. Furthermore, L-type voltage-dependent Ca2+ channels also appear to play a major role in controlling the release of glutamate in the retina[36]. Therefore, it has been hypothesized that Ca2+ channel antagonists would be effective neuroprotectants in glaucoma.
It has been hypothesized that oxidative stress plays a role in RGC death in glaucoma by damaging the trabecular meshwork, the optic nerve head, and the retina[2,37]. The term “oxidative stress”, refers to a cell's state characterized by excessive production of reactive oxygen species and/or a reduction in the antioxidant defenses responsible for their metabolism. Reactive oxygen species are not only involved in direct cytotoxic consequences leading to RGC death, but may also play roles in the cell death signaling pathway by acting as second messengers and/or modulating protein function by redox modifications of downstream effectors through enzymatic oxidation of specific amino acid residues[38]. The use of antioxidant therapy may offer unique opportunities for neuroprotective interventions aimed at the effective treatment of glaucoma.
Amyloid β peptide is constitutively produced by proteolysis of β-amyloid precursor protein and is intricately involved in the neuropathology of Alzheimer's disease. Amyloid β peptide has recently been reported to be implicated in the development of RGC apoptosis in glaucoma[17,39,40], and induces RGC apoptosis in vivo in a dose- and time-dependent manner[41]. It is likely that drugs targeting different components of the amyloid β peptide pathway would provide a therapeutic avenue for glaucoma management.
The neurotrophin deprivation hypothesis suggests that the obstruction of retrograde transport at the lamina cribrosa causes the deprivation of neurotrophic support to RGCs in glaucoma[6,42]. The neurotrophin family includes nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5[4]. Brain-derived neurotrophic factor enhances survival of RGCs in a model of excitotoxic injury[43]. Nerve growth factor was identified as being neuroprotective in the Morrison's glaucoma model by reducing RGC apoptosis through topical application[44,45]. Neurotrophic factor delivery may be a key approach in the development of potential neuroprotective glaucoma treatments.
The field of gene therapy for neuroprotection is rapidly expanding. Gene therapy involves delivery of a gene to targeted cells to cure or slow the progression of diseases. It has become a highly accessible approach for glaucoma because the trabecular meshwork, ciliary body, ciliary epithelium, Müller cells and RGCs are all appropriate target structures for gene therapy[46,47].
In addition, heat shock proteins[48] and caspase-3[49] are involved in the mechanisms believed to initiate the apoptotic cascade in glaucoma, thereby providing potential targets to rescue RGCs.
PROPOSED MULTIFUNCTIONAL DRUGS FOR NEUROPROTECTION IN GLAUCOMA
Owing to the complex etiology of glaucoma, an innovative approach to neuroprotection or neurorescue may entail the use of multifunctional pharmaceuticals that target an array of pathological pathways, each of which is believed to contribute to the cascade that ultimately leads to neuronal cell death.
Epigallocatechin gallate, a catechin-base flavonoid derived from green tea, possesses diverse pharmacological properties all of which contribute to its neuroprotective properties[50]. Besides being a powerful antioxidant[51], it also attenuates glutamate-induced cytotoxicity by decreasing calcium influx[52]. Moreover, it blocks the activation of nuclear factor-kappa B, thus preventing NOS-2 induction and consequent cytotoxic damage[53]. Epigallocatechin gallate has a protective effect on injured neurons in neurodegenerative disease, such as Alzheimer's disease and Parkinson's disease[54]. It protects RGCs from oxidative stress and ischemia/reperfusion, and its protective effect against retinal ischemia reperfusion damage is independent of any action upon IOP[50].
Bis(7)-tacrine is a promising anti-Alzheimer's dimer derived from tacrine (Figure 2A). It was originally designed as a highly potent, selective, and low cost bifunctional acetylcholinesterase inhibitor utilizing computer modeling of ligand docking with target proteins[55]. This unique compound possesses multiple physiological activities working through a multitude of mechanisms including concurrent inhibition of NMDARs[56,57], nNOS[58], L-type voltage-dependent calcium channels[59], acetylcholinesterase, and the amyloid precursor protein/β-amyloid cascade[60]. Our results confirmed that bis(7)-tacrine has neuroprotective effects against glutamate-induced RGC damage in vitro and in vivo, possibly through the drug's anti-NMDAR effects[61,62]. In glaucoma, RGCs are exquisitely sensitive to the effects of both glutamate and amyloid precursor protein, which produces a dose-dependent cell-loss both in vivo and in vitro[41,63]. We postulate that bis(7)-tacrine protects RGCs via concurrent blockade of NMDARs, voltage-dependent Ca2+ channels and nNOS in glaucomatous neurodegeneration (Figure 2B). Further studies are required to test this hypothesis.
Figure 2.
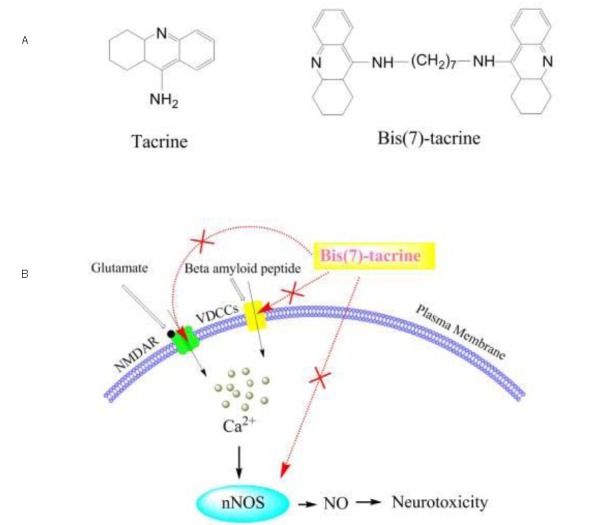
Proposed mechanism of action of bis(7)-tacrine in glaucoma neuroprotection through concurrent blockage of N-methyl-D-aspartate receptors (NMDARs), voltage-dependent Ca2+ channels (VDCCs) and neuronal nitric oxide synthase (nNOS).
(A) Chemical structure of tacrine and bis(7)-tacrine.
(B) Synergistic neuroprotection by bis(7)-tacrine via concurrent blockade of NMDAR, VDCCs and nNOS. When retinal ganglion cells are exposed to glutamate and amyloid precursor protein at toxic concentrations, excessive calcium influx mediates the subsequent biochemical events leading to neurotoxicity.
Bis(7)-tacrine concurrently blocks NMDARs, VDCCs and nNOS, thereby synergistically providing substantial neuroprotection. NO: Nitric oxide.
CONCLUSION
Currently, decreasing IOP is the only established medical treatment for glaucoma with elevated IOP, which was previously implicated as a possible primary insult in the disease[2]. Ocular or systemic administration of IOP-lowering drugs is usually the first step in glaucoma management[64]. For patients with established open-angle glaucoma (defined by the presence of optic nerve damage), reduction of IOP is effective and always recommended, irrespective of whether IOP is abnormal[65].
Lowering of IOP is accomplished by daily eye drops, laser treatment to the trabecular meshwork, or surgical treatment[1]. Pharmacological reduction of IOP is achieved by topical β-adrenoceptor blockers, topical prostaglandin analogues, topical α2-adrenoceptor agonists, topical cholinergic agonists and topical or systemic carbonic anhydrase inhibitors[3]. However, it is clear that IOP-lowering, although significantly reducing neuronal loss, does not prevent the progression of disease, because the loss of RGCs may continue, even after the IOP has been reduced[66]. Moreover, IOP lowering is not always effective and is sometimes difficult to achieve. Thus, the concept of neuroprotection of stressed RGCs has gained momentum. However, neuroprotective strategies alone are unlikely to succeed if the initial insult persists. It is therefore believed that treatment modalities that directly target both primary and secondary degeneration of the RGCs are required. Until now, there has been no neuroprotective agent indicated for the treatment of glaucoma. In the future, combination treatments targeting both IOP and neuronal injury will offer the best potential for halting the progression of RGC loss[3].
New compounds that act on multiple therapeutic targets will be highlighted. Multifunctional drugs have multiple actions that theoretically blunt a number of potential secondary insults. Future preclinical experiments in animal models of glaucoma will improve our understanding of the molecular mechanisms of action of multifunctional drugs and will reveal the potential for these drugs in clinical settings. It is probable that administration of multifunctional drugs will form an adjunct to IOP-lowering drugs in the treatment of this debilitating disease.
Footnotes
Conflicts of interest: None declared.
Funding: The original work described here was supported by the National Natural Science Foundation of China, No. 81000380; the Scientific Research Fund of Health Department, Hubei Province, China, No. QJX2010-53.
(Edited by Yang JN, Jiang CH/Qiu Y/Song LP)
REFERENCES
- [1].Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- [2].Cheung W, Guo L, Cordeiro MF. Neuroprotection in glaucoma: drug-based approaches. Optom Vis Sci. 2008;85:406–416. doi: 10.1097/OPX.0b013e31817841e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dahlmann-Noor AH, Vijay S, Limb GA, et al. Strategies for optic nerve rescue and regeneration in glaucoma and other optic neuropathies. Drug Discov Today. 2010;15:287–299. doi: 10.1016/j.drudis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- [4].Chidlow G, Wood JP, Casson RJ. Pharmacological neuroprotection for glaucoma. Drugs. 2007;67:725–759. doi: 10.2165/00003495-200767050-00006. [DOI] [PubMed] [Google Scholar]
- [5].Weinreb RN. Glaucoma neuroprotection: What is it? Why is it needed? Can J Ophthalmol. 2007;42:396–398. [PubMed] [Google Scholar]
- [6].Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 2011;22:78–86. doi: 10.1097/ICU.0b013e32834372ec. [DOI] [PubMed] [Google Scholar]
- [7].Osborne NN. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 2009;87:450–454. doi: 10.1111/j.1755-3768.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- [8].Danesh-Meyer HV, Levin LA. Neuroprotection: extrapolating from neurologic diseases to the eye. Am J Ophthalmol. 2009;148:186–191. doi: 10.1016/j.ajo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- [9].Youdim MB, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol Sci. 2005;26:27–35. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [10].Geldenhuys WJ, Youdim MB, Carroll RT, et al. The emergence of designed multiple ligands for neurodegenerative disorders. Prog Neurobiol. 2011;94:347–359. doi: 10.1016/j.pneurobio.2011.04.010. [DOI] [PubMed] [Google Scholar]
- [11].Van der Schyf CJ, Youdim MB. Multifunctional drugs as neurotherapeutics. Neurotherapeutics. 2009;6:1–3. doi: 10.1016/j.nurt.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cavalli A, Bolognesi ML, Minarini A, et al. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51:347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- [13].Valenti DA. Alzheimer's disease and glaucoma: imaging the biomarkers of neurodegenerative disease. Int J Alzheimers Dis 2011. 2010 doi: 10.4061/2010/793931. 793931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18:110–114. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- [15].Bayer AU, Keller ON, Ferrari F, et al. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer's disease and Parkinson's disease. Am J Ophthalmol. 2002;133:135–137. doi: 10.1016/s0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- [16].McKinnon SJ. Glaucoma: ocular Alzheimer's disease? Front Biosci. 2003;8:s1140–1156. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- [17].McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, et al. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci. 2002;43:1077–1087. [PubMed] [Google Scholar]
- [18].Baltmr A, Duggan J, Nizari S, et al. Neuroprotection in glaucoma - Is there a future role? Exp Eye Res. 2010;91:554–566. doi: 10.1016/j.exer.2010.08.009. [DOI] [PubMed] [Google Scholar]
- [19].Le A, Mukesh BN, McCarty CA, et al. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44:3783–3789. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- [20].Zimmermann GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- [21].Morphy R, Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005;48:6523–6543. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- [22].Yang K, Bai H, Ouyang Q, et al. Finding multiple target optimal intervention in disease-related molecular network. Mol Syst Biol. 2008;4:228. doi: 10.1038/msb.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bolognesi ML, Matera R, Minarini A, et al. Alzheimer's disease: new approaches to drug discovery. Curr Opin Chem Biol. 2009;13:303–308. doi: 10.1016/j.cbpa.2009.04.619. [DOI] [PubMed] [Google Scholar]
- [24].Witt A, Macdonald N, Kirkpatrick P. Memantine hydrochloride. Nat Rev Drug Discov. 2004;3:109–110. doi: 10.1038/nrd1311. [DOI] [PubMed] [Google Scholar]
- [25].Noorbakhsh F, Overall CM, Power C. Deciphering complex mechanisms in neurodegenerative diseases: the advent of systems biology. Trends Neurosci. 2009;32:88–100. doi: 10.1016/j.tins.2008.10.003. [DOI] [PubMed] [Google Scholar]
- [26].Stahl SM. Multifunctional drugs: a novel concept for psychopharmacology. CNS Spectr. 2009;14:71–73. doi: 10.1017/s1092852900000213. [DOI] [PubMed] [Google Scholar]
- [27].Weinreb O, Mandel S, Bar-Am O, et al. Multifunctional neuroprotective derivatives of rasagiline as anti-Alzheimer's disease drugs. Neurotherapeutics. 2009;6:163–174. doi: 10.1016/j.nurt.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- [29].Cho KJ, Kim JH, Park HY, et al. Glial cell response and iNOS expression in the optic nerve head and retina of the rat following acute high IOP ischemia-reperfusion. Brain Res. 2011;1403:67–77. doi: 10.1016/j.brainres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- [30].Kang JH, Wiggs JL, Rosner BA, et al. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010;51:971–979. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26:205–238. doi: 10.1016/j.preteyeres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- [32].Park SH, Kim JH, Kim YH, et al. Expression of neuronal nitric oxide synthase in the retina of a rat model of chronic glaucoma. Vision Res. 2007;47:2732–2740. doi: 10.1016/j.visres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- [33].Libby RT, Howell GR, Pang IH, et al. Inducible nitric oxide synthase, Nos2, does not mediate optic neuropathy and retinopathy in the DBA/2J glaucoma model. BMC Neurosci. 2007;8:108. doi: 10.1186/1471-2202-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Melena J, Osborne NN. Voltage-dependent calcium channels in the rat retina: involvement in NMDA-stimulated influx of calcium. Exp Eye Res. 2001;72:393–401. doi: 10.1006/exer.2000.0968. [DOI] [PubMed] [Google Scholar]
- [35].Brandt SK, Weatherly ME, Ware L, et al. Calcium preconditioning triggers neuroprotection in retinal ganglion cells. Neuroscience. 2011;172:387–397. doi: 10.1016/j.neuroscience.2010.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thoreson WB, Witkovsky P. Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res. 1999;18:765–810. doi: 10.1016/s1350-9462(98)00031-7. [DOI] [PubMed] [Google Scholar]
- [37].Tezel G. The immune response in glaucoma: A perspective on the roles of oxidative stress. Exp Eye Res. 2011;93:178–186. doi: 10.1016/j.exer.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tatton W, Chen D, Chalmers-Redman R, et al. Hypothesis for a common basis for neuroprotection in glaucoma and Alzheimer's disease: anti-apoptosis by alpha-2-adrenergic receptor activation. Surv Ophthalmol. 2003;48(Suppl 1):S25–37. doi: 10.1016/s0039-6257(03)00005-5. [DOI] [PubMed] [Google Scholar]
- [40].Sakamoto K, Ohki K, Saito M, et al. Histological protection by donepezil against neurodegeneration induced by ischemia-reperfusion in the rat retina. J Pharmacol Sci. 2010;112:327–335. doi: 10.1254/jphs.09302fp. [DOI] [PubMed] [Google Scholar]
- [41].Guo L, Salt TE, Luong V, et al. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104:13444–13449. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Johnson EC, Guo Y, Cepurna WO, et al. Neurotrophin roles in retinal ganglion cell survival: lessons from rat glaucoma models. Exp Eye Res. 2009;88:808–815. doi: 10.1016/j.exer.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kido N, Tanihara H, Honjo M, et al. Neuroprotective effects of brain-derived neurotrophic factor in eyes with NMDA-induced neuronal death. Brain Res. 2000;884:59–67. doi: 10.1016/s0006-8993(00)02887-0. [DOI] [PubMed] [Google Scholar]
- [44].Colafrancesco V, Parisi V, Sposato V, et al. Ocular application of nerve growth factor protects degenerating retinal ganglion cells in a rat model of glaucoma. J Glaucoma. 2011;20:100–108. doi: 10.1097/IJG.0b013e3181d787e5. [DOI] [PubMed] [Google Scholar]
- [45].Lambiase A, Aloe L, Centofanti M, et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc Natl Acad Sci U S A. 2009;106:13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Demetriades AM. Gene therapy for glaucoma. Curr Opin Ophthalmol. 2011;22:73–77. doi: 10.1097/ICU.0b013e32834371d2. [DOI] [PubMed] [Google Scholar]
- [47].Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 2010;120:3064–3072. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jeun M, Jeoung JW, Moon S, et al. Engineered superparamagnetic Mn0.5Zn0.5Fe2O4 nanoparticles as a heat shock protein induction agent for ocular neuroprotection in glaucoma. Biomaterials. 2011;32:387–394. doi: 10.1016/j.biomaterials.2010.09.016. [DOI] [PubMed] [Google Scholar]
- [49].Seki M, Soussou W, Manabe S, et al. Protection of retinal ganglion cells by caspase substrate-binding peptide IQACRG from N-methyl-D-aspartate receptor-mediated excitotoxicity. Invest Ophthalmol Vis Sci. 2010;51:1198–1207. doi: 10.1167/iovs.09-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang B, Safa R, Rusciano D, et al. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 2007;1159:40–53. doi: 10.1016/j.brainres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- [51].Xie D, Liu G, Zhu G, et al. (-)-Epigallocatechin-3-gallate protects cultured spiral ganglion cells from H2O2-induced oxidizing damage. Acta Otolaryngol. 2004;124:464–470. doi: 10.1080/00016480410018278. [DOI] [PubMed] [Google Scholar]
- [52].Lee JH, Song DK, Jung CH, et al. (-)-Epigallocatechin gallate attenuates glutamate-induced cytotoxicity via intracellular Ca modulation in PC12 cells. Clin Exp Pharmacol Physiol. 2004;31:530–536. doi: 10.1111/j.1440-1681.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- [53].Aktas O, Prozorovski T, Smorodchenko A, et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- [54].Hou RR, Chen JZ, Chen H, et al. Neuroprotective effects of (-)-epigallocatechin-3-gallate (EGCG) on paraquat-induced apoptosis in PC12 cells. Cell Biol Int. 2008;32:22–30. doi: 10.1016/j.cellbi.2007.08.007. [DOI] [PubMed] [Google Scholar]
- [55].Pang YP, Quiram P, Jelacic T, et al. Highly potent, selective, and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase. Steps toward novel drugs for treating Alzheimer's disease. J Biol Chem. 1996;271:23646–23649. doi: 10.1074/jbc.271.39.23646. [DOI] [PubMed] [Google Scholar]
- [56].Liu YW, Luo JL, Ren H, et al. Inhibition of NMDA-gated ion channels by bis(7)-tacrine: whole-cell and single-channel studies. Neuropharmacology. 2008;54:1086–1094. doi: 10.1016/j.neuropharm.2008.02.015. [DOI] [PubMed] [Google Scholar]
- [57].Luo J, Li W, Liu Y, et al. Novel dimeric bis(7)-tacrine proton-dependently inhibits NMDA-activated currents. Biochem Biophys Res Commun. 2007;361:505–509. doi: 10.1016/j.bbrc.2007.07.043. [DOI] [PubMed] [Google Scholar]
- [58].Li W, Xue J, Niu C, et al. Synergistic neuroprotection by bis(7)-tacrine via concurrent blockade of N-methyl-D-aspartate receptors and neuronal nitric-oxide synthase. Mol Pharmacol. 2007;71:1258–1267. doi: 10.1124/mol.106.029108. [DOI] [PubMed] [Google Scholar]
- [59].Fu H, Li W, Lao Y, et al. Bis(7)-tacrine attenuates beta amyloid-induced neuronal apoptosis by regulating L-type calcium channels. J Neurochem. 2006;98:1400–1410. doi: 10.1111/j.1471-4159.2006.03960.x. [DOI] [PubMed] [Google Scholar]
- [60].Li W, Mak M, Jiang H, et al. Novel anti-Alzheimer's dimer Bis(7)-cognitin: cellular and molecular mechanisms of neuroprotection through multiple targets. Neurotherapeutics. 2009;6:187–201. doi: 10.1016/j.nurt.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fang JH, Wang XH, Xu ZR, et al. Neuroprotective effects of bis(7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC Neurosci. 2010;11:31. doi: 10.1186/1471-2202-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang ZH, Liu YW, Jiang FG, et al. Bis(7)-tacrine protects retinal ganglion cells against excitotoxicity via NMDA receptor inhibition. Int J Ophthalmol. 2011;4:125–130. doi: 10.3980/j.issn.2222-3959.2011.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dreyer EB. A proposed role for excitotoxicity in glaucoma. J Glaucoma. 1998;7:62–67. [PubMed] [Google Scholar]
- [64].Alward WL. Medical management of glaucoma. N Engl J Med. 1998;339:1298–1307. doi: 10.1056/NEJM199810293391808. [DOI] [PubMed] [Google Scholar]
- [65].Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- [66].Bakalash S, Kipnis J, Yoles E, et al. Resistance of retinal ganglion cells to an increase in intraocular pressure is immune-dependent. Invest Ophthalmol Vis Sci. 2002;43:2648–2653. [PubMed] [Google Scholar]


