Abstract
The present study established a rat model of post-stroke depression using incomplete ischemia induced by unilateral carotid artery ligation in combination with solitary raising and subcutaneous injection of a small dose of reserpine. After intragastric perfusion with 45 mg/100 g, 15 mg/100 g, and 7.5 mg/100 g of Xingnao Jieyu for 7, 14 and 21 days, neuronal morphology in the frontal lobe and hippocampus was improved, depression state and voluntary behaviors were also effectively improved in rats with post-stroke depression. Moreover, the effects of Xingnao Jieyu at a dose of 45 and 15 mg/100 g were similar to the traditional antidepressant Prozac.
Keywords: Xingnao Jieyu capsule, poststroke depression, hippocampus, neuron, traditional Chinese medicine, neural regeneration
INTRODUCTION
Currently existing antidepressants have a single action pathway, and toxic and adverse effects to different degrees on the liver, kidney and heart, which frequently lead to allergies and drug withdrawal syndrome, therefore limiting their clinical application[1,2]. Thus, clinical studies have been focusing on the development of Chinese medicines with precise effects, multiple targets, and minimal side effects. Chinese medicine compounds that are used to treat post-stroke depression (PSD) are mainly divided into five types: Jieyu Anshen, Ditan Jieyu, Huoxue Jieyu, Shugan Jieyu, and Yishen Shugan prescription[3]. Treatment of PSD using modern Chinese medicine compounds pay more attention to promoting blood circulation by removing blood stasis, regulating qi to reduce phlegm, restoring consciousness and inducing resuscitation, relieving mental stress, and reinforcing kidney function. Therefore, we hypothesize that the pathogenesis of PSD involves stagnation of qi and phlegm stagnancy. Xingnao Jieyu (XNJY) has been shown to restore consciousness and relieve depression, resolving phlegm and promoting blood circulation, exhibit minimal side effects, have favorable curative effects, and have safe and reliable properties[4]. The present study further confirmed that XNJY capsule can reduce depression symptoms and improve neurological function by promoting hippocampal neuronal regeneration, and provides insights for PSD treatment.
RESULTS
Quantitative analysis of experimental animals
After 1 week of adaptation, 50 rats were randomly assigned to five equal groups: model, Prozac, XNJY capsule high-, medium- and low-dose groups. The stroke model was established by unilateral carotid artery ligation, followed by solitary raising and subcutaneous injection of a small dose of reserpine to reproduce the depression model (3 days after stroke). The model, Prozac, XNJY capsule high, medium and low dose groups were intragastrically perfused with physiological saline, Prozac and 45 mg/100 g, 15 mg/100 g, 7.5 mg/100 g XNJY capsule, respectively. All rats were included in the final analysis.
Influence of XNJY capsule on hippocampal neurons in PSD rats
Light microscopic observation showed that the size and quantity of hippocampal neurons and glial cells (round shape, with irregular margins and processes) were reduced, cells were polygon-shaped, the intercellular space was enlarged, with sparse alignment or absence of Nissl bodies in the model group. XNJY capsule and Prozac increased the size and quantity of hippocampal neurons and glial cells with regular shape and decreased intercellular space (Figure 1).
Figure 1.
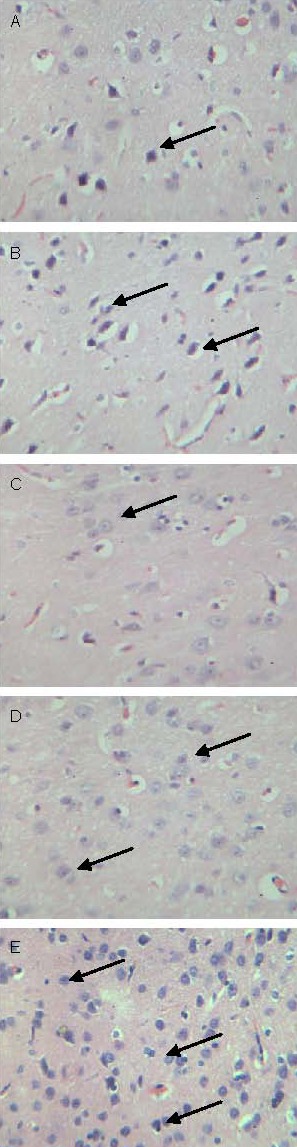
Changes in rat hippocampal neurons and glial cells (light microscopy of Nissl staining, × 100). Arrows represent neurons.
(A) The number of neurons and glial cells was reduced in the model group.
(B–E) The number of neurons and glial cells increased in the Prozac and the low-, medium- and high-dose Xingnao Jieyu capsule groups.
Quantification of the number of hippocampal neurons revealed that there were 27 ± 5 cells/100-fold field of view, 36 ± 2 cells/100-fold field of view, 32 ± 4 cells/100-fold field of view, 35 ± 6 cells/100-fold field of view, and 38 ± 3 cells/100-fold field of view in the model, Prozac, XNJY capsule low-, medium- and high-dose groups (P < 0.01 or P < 0.05 when compared to the model group), respectively. However, there was no significant difference in the number of neurons between the Prozac and the three doses of XNJY capsule groups (P > 0.05).
Influence of XNJY capsule on depression state and voluntary behavior of PSD rats
Sugar water consumption
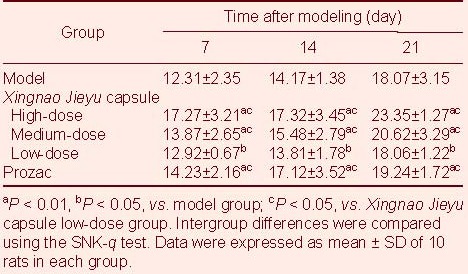
Compared with the model group, XNJY capsule and Prozac treatment significantly increased sugar water consumption of depressed rats (P < 0.01 or P < 0.05); sugar water consumption following high- and medium-dose XNJY capsule treatment, as well as Prozac treatment was significantly higher compared with low- dose XNJY capsule treatment (P < 0.05), but no differences were found between high- and medium-dose XNJY capsule treatment and the Prozac groups (P > 0.05; Table 1).
Table 1.
Changes in rat sugar consumption (mL/d)
Open-field test
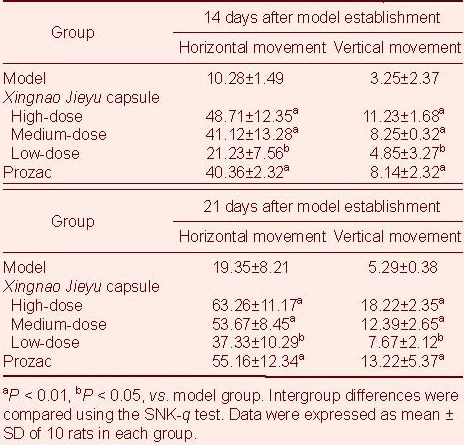
Compared with the model group, XNJY capsule and Prozac treatment significantly increased voluntary behavior of rats (P < 0.01 or P < 0.05). Moreover, XNJY capsule at high- and medium-dose and Prozac treatment significantly increased voluntary behaviors compared with low-dose XNJY treatment (P < 0.05). However, no differences were found between high- and medium-dose XNJY treatment and the Prozac group (P > 0.05; Table 2).
Table 2.
Voluntary behavior changes (score) in rats after treatment for 14 and 21 days
DISCUSSION
Previous studies have shown that XNJY capsule can effectively increase the content of in vivo monoamine neurotransmitters (5-hydroxytryptamine, noradrenaline), further regulate monoamine neurotransmitters, neurotrophic factors, hormones and corresponding signal pathways[4]. In the present study, the PSD model was established by unilateral carotid artery ligation in combination with solitary raising. Results showed that XNJY capsule can increase the size and quantity of hippocampal neurons and glial cells, and improved cell morphology, exhibiting the effects of Prozac, indicating that XNJY capsule and Prozac can promote hippocampal neuronal regeneration. The open-field test and sugar water consumption test results showed that XNJY capsule significantly increased rat voluntary behavior and sugar water consumption, and effectively improved the state of depression and behavior. Moreover, XNJY capsule at high and medium doses exhibited similar effects to Prozac. Results demonstrated that XNJY capsule can effectively treat PSD in rats and high and medium doses of XNJY capsule exhibit similar effects to the traditional antidepressant Prozac.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
The experiments were performed in the Laboratory Animal Center of Shaanxi University of Chinese Medicine from June to November 2008.
Materials
Animals
A total of 50 healthy, adult Sprague-Dawley rats (25 male and 25 female), aged 24 weeks, were provided by the Laboratory Animal Center, Xi’an Jiaotong University Medical School (license No. SCXK (Shaan) 2007-001) and housed in 150–200 lx, 12-hour day/night cycle at 22 ± 1°C, 50–70% humidity, and noise < 50 dB. Procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[5].
Drugs
XNJY capsule comprised of 10 g Rhizoma Acori Talarinowii, 10 g Radix Polygalae, 8 g Rhizoma Pinelliae, 10 g Radix Curcumae, 10 g radix bupleuri, 10 g Concretio Silicea Bambusae, 12 g Radix Salviae Miltiorrhiae, 12 g Caulis Spatholobi, and 10 g Radix Morindae Officinalis. They were decocted in water at 10 times of their total weight for 1–2 hours, filtered through gauze, followed by decoction in water at 8 times of their total weight for 1–2 hours. The solution was filtered through gauze. The filtrate of the two times solution was mixed, condensed to thick cream with a relative density of 1.25–1.30 at 50°C and mixed with starch to prepare particles. The particles were packaged into capsules. The capsule was produced by the Preparation Center, Affiliated Hospital of Shaanxi University of Chinese Medicine. Each capsule (0.3 g/capsule) contained 3.5 g of crude drug.
Hydrochloric acid Prozac capsule (20 mg per capsule) was purchased from Eli Lilly and Company, Suzhou, China (J20080016; batch number: 203216).
Methods
Establishment of PSD model
After 1 week of adaptation, the stroke model was established by unilateral carotid artery ligation[4]. At 3 days after modeling of stroke, the rats were separately housed to replicate the human solitary environment and induce a model of depression. In addition, the rats were subcutaneously injected with a small dose of reserpine (Guangdong Bangmin Pharmaceutical Co., Ltd., Guangdong, China; No. H44021892; 050213), 0.2 mg/kg, once a day for 14 days. A successful model was demonstrated when rats exhibited evident depression, low emotion, dark hair, decreased movement, body mass and appetite, diarrhea, temperature drop, decreased cold endurance, low energy metabolism, and back arching. Within the experimental time, horizontal movement and vertical movement were significantly reduced, and sugar water consumption was reduced[4].
Intragastric perfusion of XNJY capsules
After establishment of PSD, the model, Prozac, XNJY capsule high-, medium- and low-dose groups were intragastrically perfused with 2.5 mL physiological saline, Prozac (0.18 mg/100 g) and 45 mg/100 g, 15 mg/100 g, and 7.5 mg/100 g XNJY capsule, respectively, once a day (conversion according to the experimental animals and peoples use[4]). The drugs were administrated till behavioral changes were detected.
Open-field test for voluntary behavior
At 14 and 21 days after model establishment, the open-field test was performed. A self-made open box with black walls measuring 40 cm high, 80 cm long and 80 cm wide was used. The floor area comprised of 25 blocks of equal size. Horizontal movement represented the range of motion of animals and vertical motion reflected curiosity to the new environment. The number of blocks that rats passed through represented the horizontal movement score (1 square = 1 score). If rats walked along the lines, they received a score of 1 every 10 cm. The frequency of straight represented vertical movement scores (rearing): both feet of rats off the ground until they were put down was regarded as one movement, and scored 1 point. Every animal was observed for 3 minutes[6].
Sugar water consumption test for rat depression symptoms
At 7, 14 and 21 days after intervention, animals of each group were allowed free access to two kinds of water: a bottle of tap water containing 1% (w/v) sucrose and a bottle of tap water. The tap water and sucrose water consumption from 7: 00 a.m. to 7: 00 a.m. the next day was measured by weighing the bottles. Sucrose preference was compared. Sucrose preference degree (%) = sucrose water intake/(sucrose water consumption + water intake) × 100%. The average was used[7].
Hippocampal nerve cell observation
At 21 days after behavioral observations, the rats were sacrificed after anesthesia with 10% (v/v) chloral hydrate (4.0 mL/kg). Brain tissue was harvested, fixed in paraformaldehyde, sectioned (30 μm thick), defatted in 75% (v/v) alcohol for 30 minutes, stained with 0.5% (w/v) cresyl violet, dehydrated, cleared and mounted with neutral gum. Four sections from the hippocampal CA3 region within 1.5-2.5 mm posterior to bregma[8] were selected, imaged under ×100 magnification objective lens using a Motic system (Shenzhen Mashide Instrument, Guangdong, China) to observe morphological changes in hippocampal neurons. The number of hippocampal neurons in the field of view was quantified using a 25 μm2 counting plate (Shanghai Anxin Optical Equipment, Shanghai, China).
Statistical analysis
Data were expressed as the mean ± SD and analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). Intergroup differences were compared using one-way analysis of variance and the SNK-q test. Differences were considered statistically significant at an alpha level of 0.05.
Acknowledgements:
We thank Xiaoping Xu, Department of Pharmacology, Shaanxi University of Chinese Medicine, Duosi Xu, Department of Shaanxi University of Chinese Medicine for technical support.
Footnotes
Conflicts of interest: None declared.
Funding: This project was supported by the Major Program of “13115” Science and Technology Innovation Project (Preclinical study of Xingnao Jieyu capsule), No. 2010ZDKG-65.
Ethical approval: This study received permission from the Animal Ethics Committee of Shaanxi University of Chinese Medicine, China
(Edited by Huang SJ, Zhao Y/Su LL/Wang L)
REFERENCES
- [1].Robinson RG. Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry. 2003;54(3):376–387. doi: 10.1016/s0006-3223(03)00423-2. [DOI] [PubMed] [Google Scholar]
- [2].Hackett ML, Yapa C, Parag V, et al. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(6):1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- [3].Yan YM, Liu L, Fan WT. Effects of Xingnao Jieyu capsule on monoamine neurotransmitters in PSD rat. Shanxi Zhongyi. 2006;26(9):1150–1151. [Google Scholar]
- [4].Fan WT, Wang Q. Establishment of post-stroke depression animal model. Changchun Zhongyiyao Daxue Xuebao. 2007;23(1):15–17. [Google Scholar]
- [5].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [6].Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5(2):247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- [7].Papp M, Klimek V, Willner P. Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology (Berl) 1994;115(4):441–446. doi: 10.1007/BF02245566. [DOI] [PubMed] [Google Scholar]
- [8].Purves D, Augustine GJ, Fitzpatrick D, et al. 2nd ed. Sunderland: Sinauer Associates; 2001. Neuroscience. [Google Scholar]


