Abstract
Chronic kidney disease (CKD) is a health care problem with increasing prevalence worldwide. Pain management represents one of the challenges in providing perioperative care for this group of patients. Physicians from different specialties may be involved in pain management of CKD patients, especially in advanced stages. It is important to understand the clinical staging of kidney function in CKD patients as the pharmacotherapeutic pain management strategies change as kidney function becomes progressively impaired. Special emphasis should be placed on dose adjustment of certain analgesics as well as prevention of further deterioration of renal function that could be induced by certain classes of analgesics. Chronic pain is a common finding in CKD patients which may be caused by the primary disease that led to kidney damage or can be a direct result of CKD and hemodialysis. The presence of chronic pain in some of the CKD patients makes postoperative pain management in these patients more challenging. This review focuses on the plans and challenges of postoperative pain management for patient at different stages of CKD undergoing surgical intervention to provide optimum pain control for this patient population. Further clinical studies are required to address the optimal medication regimen for postoperative pain management in the different stages of CKD.
Keywords: Chronic, end stage, kidney failure, pain, specialist
Introduction
Chronic kidney disease (CKD) is a health care problem with increasing prevalence worldwide. Anesthetic and postoperative pain management for these patients can be challenging. Patients present with comorbid medical problems that may be the cause or consequences of their failed kidney function. Due to the paucity of large clinical trials in some aspects of pain management in CKD, published guidelines might depend on specific institutional experience or through applying the already available data regarding the changes in pharmacokinetics of pain medications in this group of patients. This article will focus on identifying the challenges of pain management for CKD patient undergoing surgical intervention and explores avenues to overcome these difficulties through simplifying some of the published guidelines in this field to provide optimum pain control for this patient population.
Definition and Staging of Chronic Kidney Disease
The National Kidney Foundation Kidney Disease Outcome Quality Initiative (K/DOQI) Advisory Board (2002) provides a standard definition for CKD. Patients with CKD should have either a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 for ≥3 months or structural/functional kidney damage with or without changes in GFR.[1,2,3] Evaluation of kidney function is more dependent on GFR or the presence of other markers of kidney damage rather than a single serum creatinine (SCr) reading.[4] GFR can be estimated by using SCr using the Cockcroft–Gault equation to calculate the creatinine clearance (CCr).[5] Alternatively, it can be estimated through the Modification of Diet in Renal Disease (MDRD) study equation to provide the GFR where age is in years, and weight is in kilogram.[6,7] In recent years, researchers have developed a modified version of the MDRD called the CKD epidemiology collaboration equation.[8] This equation is more useful at estimating kidney function in individuals with less severe disease and it is replacing the MDRD study equation in routine clinical use
The K/DOQI Advisory Board has divided the progression of CKD into five stages [Table 1]. Stage 1 is defined as kidney damage with normal or increased GFR, while Stage 2 is defined as a mild reduction in renal function and GFR. At these stages, patients may be asymptomatic and kidney disease may be diagnosed incidentally or found during investigations for other illnesses such as diabetes mellitus. Stages 3 and 4 are associated with moderate to severe impairment of renal function and reduction in GFR. These patients may have some uremic symptoms related to the impaired renal function. Stage 5 is end-stage renal disease (ESRD) and patients at this stage require dialysis or renal replacement therapy (RRT).[1,2]
Table 1.
Stages of CKD

It is important to understand the clinical staging of kidney function in CKD patients undergoing surgery to reduce possible adverse effects of anesthetics and analgesics, and to understand the limitations and difficulties in managing post-operative pain.
Challenges in Postoperative Pain Management in Chronic Kidney Disease
Management issues in postoperative pain management for CKD patients may include one or more of the following.
Prevention of further renal damage
Special emphasis should be placed on preventing further deterioration of renal function as well as protection of existing renal function in patients with moderate to severe impairment from the effects of anesthetics and pain medications. Careful consideration should be given to following up on postoperative renal function for these patients due to susceptibility of further deterioration in kidney function.[9,10] For example, analgesics such as nonsteroidal antiinflammatory drugs (NSAIDs) can contribute to a reduction of the residual renal function in CKD.[11]
Dose adjustment
For patients with significant reductions in GFR, dose adjustment and avoidance of certain analgesics may be necessary due to an alteration in the pharmacokinetics and pharmacodynamics of several analgesic agents and their metabolites. CKD patients are at increased risk for adverse effects from associated comorbidities, increased drug sensitivity, reduction in body mass, a small margin between analgesia, and toxicity, and drug accumulation due to impaired excretion.[12] These pharmacokinetic and pharmacodynamic changes depend on the pharmacological agent itself, the stage of renal impairment, and whether the patient is undergoing dialysis.[13,14] Furthermore, high percentages of patients with CKD are elderly and this may additionally increase the sensitivity of these patients to pain medications.[2]
The presence of chronic pain
Chronic pain is a common finding in CKD patients. Pain may be caused by the primary disease that led to kidney damage or can be a direct result of CKD and hemodialysis [Table 2].[12,13,14] Chronic pain is reported in 40-50% of hemodialysis patients and more than 80% of those patients have experienced moderate to severe pain.[12,13,14]
Table 2.
Cause of chronic pain in CKD

The role of regional and neuraxial analgesia in chronic kidney disease
The use of peripheral nerve blocks or neuraxial techniques can play a role in avoiding the adverse effects of both anesthetics and analgesics. Concerns regarding the use of these methods for CKD patients may arise in patients undergoing dialysis when platelet number and function may be deranged, in addition to the frequent use of anticoagulants during dialysis. Although peripheral nerve blocks are used to assist in providing postoperative pain control in ESRD, concern still exists regarding the use of epidural techniques for these patients.[15,16,17] Hemodialysis has been reported as a risk factor for developing epidural hematoma in hemodialysis patients.[15,16] However, studies have reported the successful use of epidural analgesia for both obstetrical and nonobstetrical procedures in dialysis patients without developing epidural hematoma.[18,19,20] Due to the small sample sizes of these reports, it is difficult to provide an estimate of the rate of this complication.
Pain Management in Chronic Kidney Disease Stage 1
Patients in CKD-Stage 1 usually have normal renal function (GFR ≥90 mL/min/1.73 m2). A structural abnormality in the kidney is present that is typically diagnosed incidentally during radiological investigations, such as the presence of kidney cyst.[1,2,4] Postoperative and posttraumatic pain management for these patients should not differ from other patients without kidney disease.[9,10] Analgesic plans should consider using neuraxial or peripheral nerve blockade whenever possible. A multimodal pain management regimen is preferably to be used for both intraoperative and postoperative analgesia. Acetaminophen, NSAIDs, or specific cyclooxygenase-2 inhibiters should be considered as analgesic foundations and be used around the clock unless otherwise contraindicated.[21] These simple analgesics may be enough to treat mild to moderate postoperative pain as well as to reduce opioid requirements and related side effects.[22,23,24]
NSAIDs are valuable analgesic adjuncts and should not be withheld from these patients, as there is an absence of evidence to suggest that short-term therapy predisposes to any further chronic renal impairment.[25] Other adjuvants can be used to improve perioperative pain control, reduce the dose of opioids, and minimize the possibility of progression of acute postsurgical or posttrauma pain to chronic pain. These medications can include tramadol and anticonvulsant medications such as gabapentin or pregabalin, which can improve pain management, especially for those patients with a neuropathic pain component.[12,26,27]

Pain Management in Chronic Kidney Disease Stage 2
In CKD — Stage 2, patients have a mild degree of impairment in renal function (GFR 60-89 mL/min/1.73 m2). The level of renal function for these patients is sufficient for excretion of the drugs and their metabolites with no need for dose adjustment of pain medications.[1,2,3,4]
There are two main considerations for patients with Stage 2 CKD. Patients should be monitored for further deterioration of renal function due to anesthetic or other perioperative events such as bleeding, dehydration, or prolonged hypotension. Such events that alter renal function may warrant readjustment of the doses for some analgesics.[25] Another important consideration is the possible effect of NSAIDs on the kidney causing further impairment.
Prostaglandins mediate the compensatory vasodilatation of the afferent arterioles that feed the glomerulus to maintain the GFR states of hypovolemia and hypotension.[25,28] NSAIDs cause depletion of renal vasodilator prostaglandins and allow an unopposed vasoconstriction to occur. In those with underlying renal disease or effective volume depletion, the addition of NSAIDs may catastrophically diminish renal blood flow.[28,29] In general, the triad of NSAIDs, hypotension and angiotensin converting enzyme(ACE) inhibitors can severely impair the GFR and renal function. However, in most cases the effect can be transient with no clear evidence for long-term impairment of renal function.[25,28,29,30] The risk of using NSAIDs in this group of patients should be balanced against the benefit. If NSAIDs must be used, they should be limited to the shortest duration possible and renal function should be monitored closely. NSAIDs should be avoided in patients with additional factors that can impair renal function such as advanced age, diabetes, use of ACE-inhibitors and perioperative dehydration or hypotension.[25,29,30] cyclooxygenase-2 inhibitors, like other NSAIDs, must also be used cautiously in these patients.[31]

Pain Management in Chronic Kidney Disease Stage 3 and 4
Patients with Stage 3 and 4 CKD have moderate to severe impairment of renal function with GFR between 15 and 59 mL/min/1.73 m2. The clinical utility of most analgesic drugs is altered in these patients due to altered clearance of the parent drugs and their therapeutically active or toxic metabolites. NSAIDs may also worsen the preexisting renal impairment in these patients.[1,2,9] Regional techniques should be considered whenever possible as they can help to avoid or reduce exposure to systemically administered analgesics.[21,32] Analgesic management principles should include avoidance of NSAIDs, reduction of analgesic drug doses according to the GFR or CCr, and close monitoring of drug side effects such as sedation.[33]
The use of opioids for patients with moderate to severe renal insufficiency poses one of the largest concerns for clinicians. Although dose restriction is recommended for most, if not all, opioid analgesics in this patient population, making a fixed algorithm for these medications in relation to GFR is not always feasible for two reasons. First, opioids undergo hepatic metabolism as a main route of elimination, but some opioids still have active or toxic metabolites that need to be excreted through kidney. The second reason is the increased sensitivity of the central nervous system in CKD patients to opioids in a fashion, which is not fully correlated with GFR.[12,33] The controlled released forms of opioid medications carry higher risks of unwanted side effects and toxicity in patients with renal insufficiency. Controlled release formulations are preferably avoided especially for patients with Stage 4-CKD (GFR <30 mL/min/1.73 m2).
The use of other adjuvants such as antiepileptic's can cause serious side effects in patients with compromised renal function and dose adjustment must be considered. Gabapentin and pregabalin should be used with caution in these patients and only when they are indicated such as for cases of neuropathic pain. Liberal administration of gabapentinoids may increase the risk of over sedation and even coma. These agents do not undergo hepatic metabolism and are excreted solely by the kidney. A reduction of 50% of the dose for each 50% decline in GFR or CCr, and increasing the time interval between the doses is advised.[26,34,47,48,49,50]
Opioids in Advanced, Nondialysis Dependent Chronic Kidney Disease
The degree to which analgesic drug alteration is required for CKD patients largely depends on whether the drug has active metabolites that are dependent on the kidney for excretion. Understanding metabolism of the commonly used analgesic drugs for postoperative pain management helps guide decisions about the type and dose of these agents to be used.[13,33] Alfentanil and Fentanyl exhibit a safe pharmacological profile for patients with renal impairment. Both are metabolized by the liver producing no active or toxic metabolites. Less than 10% of the parent drug is excreted unchanged in the urine. There is no dose modification required for boluses, but caution is advised with repeated boluses or continuous infusion, as there may be a slight plasma accumulation during advanced stages of renal impairment.[30,34]
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G). These metabolites are renally excreted and 5-10% of morphine is eliminated unchanged as the parent drug. Unlike M6G, M3G has no analgesic activity. M3G is associated with hyperalgesia and neurotoxicity when accumulated in patients with severe renal impairment. These patients will be more susceptible to develop side effects from morphine such as respiratory depression, nausea, vomiting, and myoclonus. For patients with GFR of 50 mL/min/1.73 m2 or less, morphine dose reduction should be considered by more than 50% and is even best avoided when possible. The risk of toxicity is much higher with GFR <30 mL/min/1.73 m2 and morphine avoidance becomes highly recommended.[30,35,36,37]
Meperidine (pethidine) is deemed comparatively worse than morphine when considering side effects due to the accumulation of norpethidine, which is a renally excreted neurotoxic metabolite. Patients will be at risk for seizures, especially with repeated doses of meperidine and this drug is, therefore, not recommended for this group of patients.[36,37]
Hydromorphone, which is a morphine analogue, is better tolerated in patients with renal insufficiency. However, metabolism does lead to hydromorphone-3-glucoronide (H3G), which can have similar neurotoxic effects as M3G when accumulated in the plasma. In cases of moderate renal impairment, H3G levels in the plasma can be 100 times that of the parent drug. Though hydromorphone is more potent than morphine, less-drug is needed to provide similar analgesic responses. Therefore the overall production and neurotoxic effects of H3G aremay be comparatively less than that of M3G, despite its greater potency when compared to M3G.[38,39] Unlike morphine, hydromorphone does not have an analgesically active 6-glucuronide metabolite, which may decrease the risk of developing respiratory depression in this population. Accumulation of both H3G and M3G in ESRD patients between hemodialysis sessions has been associated with increase hyperalgesia but the effect of H3G is deemed less than that of M3G.[40,41] Although authors agree that hydromorphone is relatively safer than morphine in renal insufficiency, dose adjustment depending on GFR is still recommended.[42]
Codeine should be avoided in this patient population since it will be converted in the liver to morphine and its metabolites (M3G, M6G). In those who are rapid metabolizers, too much morphine can be produced leading to a higher risk of toxicity that can occur even after trivial doses.[36,42] Oxycodone is metabolized by the liver with less than 10% excreted unchanged in the urine. There are reports of toxicity due to the accumulation of both the parent compound and metabolites in patients with renal insufficiency and thus should be used with caution. There is little evidence regarding the use of oxycodone for acute postoperative pain as it is more commonly used for chronic pain.[37,42,43]
Analgesic efficacy of tramadol requires metabolism to its active metabolite, O-desmethyltramadol (M1), by the liver in a similar fashion as codeine. Failure of excretion of this metabolite through the kidney can lead to accumulation causing serious side effects such as sedation and seizure activity.[44] Co-administration of serotonin re-uptake inhibitors and other serotonergic agents may increase the possibility of developing seizures. Lowering the maximum daily dose and increasing the interval between doses are important steps in using this analgesic medication safely in patients with renal insufficiency.[44,45] It is preferable to refer to acute pain service dosage guidance for analgesics and adjuvants [Table 3].
Table 3.
Acute pain service dosage guidance for analgesics and adjuvants used in renal insufficiency

Ketamine in Advanced, Nondialysis Dependent Chronic Kidney Disease
Ketamine is an N-methyl-d-aspartate receptor antagonist that is commonly used as an adjunct for the treatment of acute postoperative or posttraumatic pain to improve pain scores and reduce opioid consumption by approximately 30-50%.[46] Certain patients seem to benefit more from the addition of ketamine, including those with chronic neuropathic pain, opioid dependence or tolerance and acute hyperalgesia.[47] 8% of administered ketamine is metabolized by the liver forming norketamine, which possess only 20-30% of the potency of ketamine. Norketamine is then hydroxylated into a water-soluble metabolite excreted by the kidney.[48] Most clinicians believe that dose modification for ketamine is not required for patients with decreased renal function.[48,49] It can be administered in a variety of fashions such as adding it to opioids used in patient controlled analgesia (PCA) pumps, administering it through continuous infusions or even providing patients oral divided doses.[46,48,49]

Pain Management in End Stage Renal Disease (Chronic Kidney Disease Stage 5)
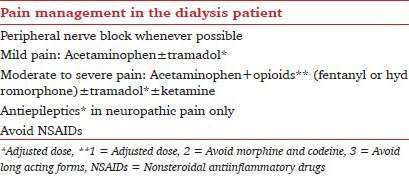
Postoperative pain management in the nondialysis ESRD patient follows the same rules as delineated for CKD-Stage4. For dialysis dependent patients, the development of the acute-on-chronic pain may also influence the quality of patient care and pain management. Approximately 50% of dialysis patients experience chronic pain that is rated as severe. Chronic pain management for these patients is beyond the scope of this review. Pain can be further complicated by comorbid depression in a high proportion of patients as well as blunted cognitive function which can make interpreting their pain more difficult. Cyclical changes in the plasma concentration of most analgesics before and after dialysis increases the need for dynamic adjustment of doses to balance the risk of toxicity before dialysis and escalation of pain after dialysis.[1,2,12,13,14]
Acetaminophen is considered the safest analgesic agent in ESRD and should be used routinely as an analgesic foundation. With chronic use, however, acetaminophen itself may cause nephrotoxicity. Although some authors suggest there is no need to adjust the acetaminophen maximum daily dose in patients undergoing intermittent hemodialysis, the 4 g/day recommended maximum dose may not be applicable for the majority of patients due to the presence of other factors that increase the risk of toxicity. In addition to renal failure, the presence of malnutrition, underlying liver impairment, or excessive alcohol consumption are risk factors for serious complications of acetaminophen related toxicity for these patients.[30,50,51,52]
There is no role for NSAIDS in the postoperative pain management in ESRD due to the high risk of serious side effects in this population [Table 4].[30,51,53] Some exceptions may be accepted for short-term NSAIDs usage in ESRD such as for those patients with acute, painful gout.[14]
Table 4.
Complications of NSAIDs in patient with ESRD

The approach to the use of opioid analgesics for dialysis patients is summarized in Table 5.[12,13,30,33,34] The use of fentanyl, alfentanil and hydromorphone are relatively safe in dialysis patients but doses should be adjusted to minimize the risk of respiratory depression.[30,34] Physicians taking care of postoperative patients may need to take into consideration the possibility of lingering anesthetic agents and the accumulation of other analgesic adjuvants before dialysis. The general condition of dialysis patients with comorbid illnesses may also aggravate the risk of opioid complications.[30,34,51,52] Morphine should be avoided due to the accumulation of toxic metabolite M3G.[30,35,36,37] Codeine, which will be converted to morphine, should also be avoided for the same reasons.[36,42] The old concept of poorly dialyzable M3G and M6G has been changed since new studies found that hemodialysis reduces the concentration of glucoronidated metabolites of morphine after dialysis.[54]
Table 5.
Opioids safety in patient with ESRD
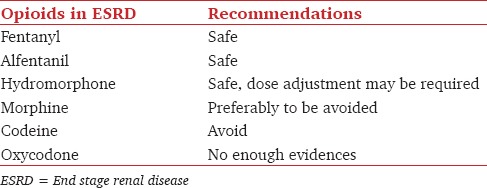
Meperidine (pethidine) again needs to be avoided due to the accumulation of norpethidine, which is neurotoxic as well.[36,37] Recommendations for the use of tramadol in dialysis dependent patients include lowering the maximum daily dose and increasing the interval between doses. These strategies help to avoid the risks of sedation, respiratory depression and seizure from accumulation of its metabolite.[44,45]
Adjuvant medications are helpful for improving pain scores, reducing opioid doses and treating neuropathic components of pain in dialysis patients. The role of ketamine in treating dialysis and palliative care patients with chronic pain is well established.[48,49,55] Ketamine plays an important role in managing acute and acute-on-chronic pain and can be administered through various routes, or combined with opioids in PCA pumps.[43,45,52]
Anticonvulsant analgesics may be required for the treatment of neuropathic pain. These medications can increase the risk of sedation in dialysis patients with accumulation between dialysis sessions. Withdrawal and escalation of pain can occur after dialysis due to the wash out of these solely renal excreted medications from patients’ plasma. A small daily dose can be used on nondialysis day with an additional loading dose immediately after dialysis.[26,34,56,57,58,59]
Conclusion
Pharmacotherapy pain management strategies for patients with CKD change as kidney function becomes progressively impaired. When devising a strategy, physicians must strive to protect the kidney from further damage as well as avoid developing serious side effects due to accumulations of analgesic agents or their metabolites. Understanding the pharmacokinetics of analgesic agents may help to predict their tolerability in patients with CKD. Further clinical studies are required to address the optimal medication regimen that can be used for postoperative pain management in the different stages of CKD, including hemodialysis.
Acknowledgments
The authors would like to thank Dr. John Penning (Director, Acute Pain Service, The Ottawa Hospital) and Dr. Naveen Eipe (Staff Anesthesiologist, The Ottawa Hospital) for sharing their experiences in acute pain management with the authors. Authors also like to thank Dr. Pat Morley-Forster (Medical Director, SJHC Pain Management Program, Western University) for her help to improve this review.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 2.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Bailie GR, Uhlig K, Levey AS. Clinical practice guidelines in nephrology: Evaluation, classification, and stratification of chronic kidney disease. Pharmacotherapy. 2005;25:491–502. doi: 10.1592/phco.25.4.491.61034. [DOI] [PubMed] [Google Scholar]
- 4.Khwaja A, Throssell D. A critique of the UK NICE guidance for the detection and management of individuals with chronic kidney disease. Nephron Clin Pract. 2009;113:c207–13. doi: 10.1159/000235240. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Greene T, Kusek JW, Beck GL MDRD Study Group. A simplified equation to predict glomerular filtration rate from serum creatinine (abstract) J Am Soc Nephrol. 2000;11(Suppl):155A. [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sear JW. Kidney dysfunction in the postoperative period. Br J Anaesth. 2005;95:20–32. doi: 10.1093/bja/aei018. [DOI] [PubMed] [Google Scholar]
- 10.Eilers H, Liu KD, Gruber A, Niemann CU. Chronic kidney disease: Implications for the perioperative period. Minerva Anestesiol. 2010;76:725–36. [PubMed] [Google Scholar]
- 11.Perazella MA. Drug-induced nephropathy: An update. Expert Opin Drug Saf. 2005;4:689–706. doi: 10.1517/14740338.4.4.689. [DOI] [PubMed] [Google Scholar]
- 12.Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: A review of analgesics in renal failure. J Nephrol. 2011;24:35–40. doi: 10.5301/jn.2010.1330. [DOI] [PubMed] [Google Scholar]
- 13.Williams A, Manias E. A structured literature review of pain assessment and management of patients with chronic kidney disease. J Clin Nurs. 2008;17:69–81. doi: 10.1111/j.1365-2702.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 14.Davison SN. Pain in hemodialysis patients: Prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42:1239–47. doi: 10.1053/j.ajkd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Basta M, Sloan P. Epidural hematoma following epidural catheter placement in a patient with chronic renal failure. Can J Anaesth. 1999;46:271–4. doi: 10.1007/BF03012609. [DOI] [PubMed] [Google Scholar]
- 16.Trainor D, Borthwick E, Ferguson A. Perioperative management of the hemodialysis patient. Semin Dial. 2011;24:314–26. doi: 10.1111/j.1525-139X.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- 17.Laskowski IA, Muhs B, Rockman CR, Adelman MA, Ranson M, Cayne NS, et al. Regional nerve block allows for optimization of planning in the creation of arteriovenous access for hemodialysis by improving superficial venous dilatation. Ann Vasc Surg. 2007;21:730–3. doi: 10.1016/j.avsg.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Kazimirov VG, Pisariuk SN, Perlin DV. Advantages of epidural anesthesia in patients with end-stage chronic renal failure. Anesteziol Reanimatol. 1995;4:63–6. [PubMed] [Google Scholar]
- 19.Hadimioglu N, Ertug Z, Bigat Z, Yilmaz M, Yegin A. A randomized study comparing combined spinal epidural or general anesthesia for renal transplant surgery. Transplant Proc. 2005;37:2020–2. doi: 10.1016/j.transproceed.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Dhir S, Fuller J. Case report: Pregnancy in hemodialysis-dependent end-stage renal disease: Anesthetic considerations. Can J Anaesth. 2007;54:556–60. doi: 10.1007/BF03022320. [DOI] [PubMed] [Google Scholar]
- 21.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–73. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 22.Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst Rev. 2008;4:CD004602. doi: 10.1002/14651858.CD004602.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derry S, Moore RA. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev. 2013;10:CD004233. doi: 10.1002/14651858.CD004233.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: A systematic review. Br J Anaesth. 2011;106:292–7. doi: 10.1093/bja/aeq406. [DOI] [PubMed] [Google Scholar]
- 25.Lee A, Cooper MG, Craig JC, Knight JF, Keneally JP. Effects of nonsteroidal anti-inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database Syst Rev. 2007;2:CD002765. doi: 10.1002/14651858.CD002765.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dauri M, Faria S, Gatti A, Celidonio L, Carpenedo R, Sabato AF. Gabapentin and pregabalin for the acute post-operative pain management. A systematic-narrative review of the recent clinical evidences. Curr Drug Targets. 2009;10:716–33. doi: 10.2174/138945009788982513. [DOI] [PubMed] [Google Scholar]
- 27.Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: A combined systematic review and meta-analysis. Anesth Analg. 2012;115:428–42. doi: 10.1213/ANE.0b013e318249d36e. [DOI] [PubMed] [Google Scholar]
- 28.Huerta C, Castellsague J, Varas-Lorenzo C, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45:531–9. doi: 10.1053/j.ajkd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Stürmer T, Elseviers MM, De Broe ME. Nonsteroidal anti-inflammatory drugs and the kidney. Curr Opin Nephrol Hypertens. 2001;10:161–3. doi: 10.1097/00041552-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: A review of available evidence. Am J Kidney Dis. 2003;42:217–28. doi: 10.1016/s0272-6386(03)00645-0. [DOI] [PubMed] [Google Scholar]
- 31.Giovanni G, Giovanni P. Do non-steroidal anti-inflammatory drugs and COX-2 selective inhibitors have different renal effects? J Nephrol. 2002;15:480–8. [PubMed] [Google Scholar]
- 32.Murphy EJ. Acute pain management pharmacology for the patient with concurrent renal or hepatic disease. Anaesth Intensive Care. 2005;33:311–22. doi: 10.1177/0310057X0503300306. [DOI] [PubMed] [Google Scholar]
- 33.Murtagh FE, Chai MO, Donohoe P, Edmonds PM, Higginson IJ. The use of opioid analgesia in end-stage renal disease patients managed without dialysis: Recommendations for practice. J Pain Palliat Care Pharmacother. 2007;21:5–16. [PubMed] [Google Scholar]
- 34.Davison SN. The prevalence and management of chronic pain in end-stage renal disease. J Palliat Med. 2007;10:1277–87. doi: 10.1089/jpm.2007.0142. [DOI] [PubMed] [Google Scholar]
- 35.Felden L, Walter C, Harder S, Treede RD, Kayser H, Drover D, et al. Comparative clinical effects of hydromorphone and morphine: A meta-analysis. Br J Anaesth. 2011;107:319–28. doi: 10.1093/bja/aer232. [DOI] [PubMed] [Google Scholar]
- 36.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613–24. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies G, Kingswood C, Street M. Pharmacokinetics of opioids in renal dysfunction. Clin Pharmacokinet. 1996;31:410–22. doi: 10.2165/00003088-199631060-00002. [DOI] [PubMed] [Google Scholar]
- 38.Murray A, Hagen NA. Hydromorphone. J Pain Symptom Manage. 2005;29:S57–66. doi: 10.1016/j.jpainsymman.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Wright AW, Mather LE, Smith MT. Hydromorphone-3-glucuronide: A more potent neuro-excitant than its structural analogue, morphine-3-glucuronide. Life Sci. 2001;69:409–20. doi: 10.1016/s0024-3205(01)01133-x. [DOI] [PubMed] [Google Scholar]
- 40.Juba KM, Wahler RG, Daron SM. Morphine and hydromorphone-induced hyperalgesia in a hospice patient. J Palliat Med. 2013;16:809–12. doi: 10.1089/jpm.2011.0502. [DOI] [PubMed] [Google Scholar]
- 41.Davison SN, Mayo PR. Pain management in chronic kidney disease: The pharmacokinetics and pharmacodynamics of hydromorphone and hydromorphone-3-glucuronide in hemodialysis patients. J Opioid Manag. 2008;4:335–6. 339. [PubMed] [Google Scholar]
- 42.Murtagh FE, Chai MO, Donohoe P, Edmonds PM, Higginson IJ. The use of opioid analgesia in end-stage renal disease patients managed without dialysis: Recommendations for practice. J Pain Palliat Care Pharmacother. 2007;21:5–16. [PubMed] [Google Scholar]
- 43.Lugo RA, Kern SE. The pharmacokinetics of oxycodone. Postgrad Med. 2009;121:91–102. [Google Scholar]
- 44.Afshari R, Ghooshkhanehee H. Tramadol overdose induced seizure, dramatic rise of CPK and acute renal failure. J Pak Med Assoc. 2009;59:178. [PubMed] [Google Scholar]
- 45.Gardner JS, Blough D, Drinkard CR, Shatin D, Anderson G, Graham D, et al. Tramadol and seizures: A surveillance study in a managed care population. Pharmacotherapy. 2000;20:1423–31. doi: 10.1592/phco.20.19.1423.34854. [DOI] [PubMed] [Google Scholar]
- 46.Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2006;1:CD004603. doi: 10.1002/14651858.CD004603.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Chazan S, Ekstein MP, Marouani N, Weinbroum AA. Ketamine for acute and subacute pain in opioid-tolerant patients. J Opioid Manag. 2008;4:173–80. doi: 10.5055/jom.2008.0023. [DOI] [PubMed] [Google Scholar]
- 48.Aroni F, Iacovidou N, Dontas I, Pourzitaki C, Xanthos T. Pharmacological aspects and potential new clinical applications of ketamine: Reevaluation of an old drug. J Clin Pharmacol. 2009;49:957–64. doi: 10.1177/0091270009337941. [DOI] [PubMed] [Google Scholar]
- 49.Capel MM, Jenkins R, Jefferson M, Thomas DM. Use of ketamine for ischemic pain in end-stage renal failure. J Pain Symptom Manage. 2008;35:232–4. doi: 10.1016/j.jpainsymman.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 50.O’Connor NR, Corcoran AM. End-stage renal disease: Symptom management and advance care planning. Am Fam Physician. 2012;85:705–10. [PubMed] [Google Scholar]
- 51.Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: A review of available evidence. Am J Kidney Dis. 2003;42:217–28. doi: 10.1016/s0272-6386(03)00645-0. [DOI] [PubMed] [Google Scholar]
- 52.Toussaint K, Yang XC, Zielinski MA, Reigle KL, Sacavage SD, Nagar S, et al. What do we (not) know about how paracetamol (acetaminophen) works? J Clin Pharm Ther. 2010;35:617–38. doi: 10.1111/j.1365-2710.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 53.Kuo HW, Tsai SS, Tiao MM, Liu YC, Lee IM, Yang CY. Analgesic use and the risk for progression of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2010;19:745–51. doi: 10.1002/pds.1962. [DOI] [PubMed] [Google Scholar]
- 54.Molanaei H, Carrero JJ, Heimbürger O, Nordfors L, Lindholm B, Stenvinkel P, et al. Influence of the CYP2D6 polymorphism and hemodialysis on codeine disposition in patients with end-stage renal disease. Eur J Clin Pharmacol. 2010;66:269–73. doi: 10.1007/s00228-009-0759-8. [DOI] [PubMed] [Google Scholar]
- 55.Okon T. Ketamine: An introduction for the pain and palliative medicine physician. Pain Physician. 2007;10:493–500. [PubMed] [Google Scholar]
- 56.Dogukan A, Aygen B, Berilgen MS, Dag S, Bektas S, Gunal AI. Gabapentin-induced coma in a patient with renal failure. Hemodial Int. 2006;10:168–9. doi: 10.1111/j.1542-4758.2006.00089.x. [DOI] [PubMed] [Google Scholar]
- 57.Gajraj NM. Pregabalin: Its pharmacology and use in pain management. Anesth Analg. 2007;105:1805–15. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 58.Randinitis EJ, Posvar EL, Alvey CW, Sedman AJ, Cook JA, Bockbrader HN. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol. 2003;43:277–83. doi: 10.1177/0091270003251119. [DOI] [PubMed] [Google Scholar]
- 59.Miller A, Price G. Gabapentin toxicity in renal failure: The importance of dose adjustment. Pain Med. 2009;10:190–2. doi: 10.1111/j.1526-4637.2008.00492.x. [DOI] [PubMed] [Google Scholar]


