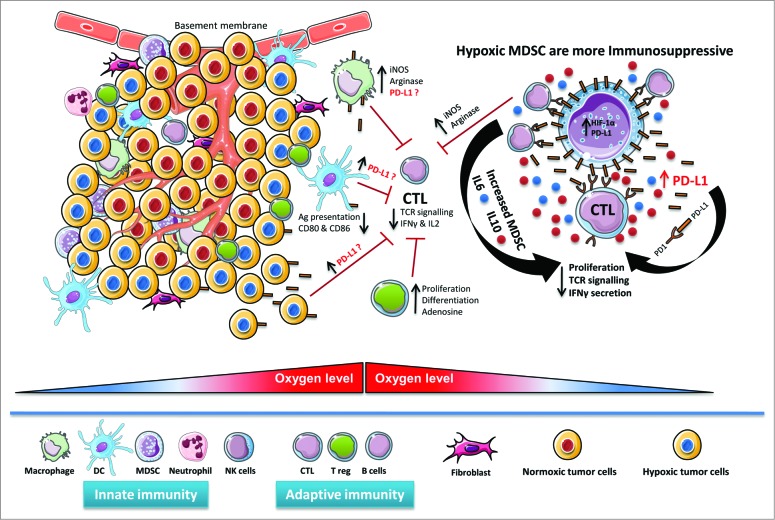
Figure 1.
Hypoxic regulation of PD-L1 expression in the tumor microenvironment. Hypoxic zones in tumors attract diverse immunosuppressive myeloid cells such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), as well as lymphoid cells, such as regulatory T cells (Tregs) and cytotoxic T lymphocytes (CTLs). By virtue of hypoxia-inducible factor 1α (HIF-1α), hypoxia upregulates programmed cell death ligand 1 (PD-L1) expression on MDSCs, macrophages, antigen (Ag)-presenting dendritic cells and malignant cells in the tumor microenvironment. The potential contribution of increased PD-L1 on the surface of MDSCs, macrophages, dendritic cells and cancer cells in regulating immunosuppression within the hypoxic tumor microenvironment remains largely unknown. Hypoxic MDSC's are more immunosuppressive due to enhanced arginase and nitric oxide (iNOS) production. Hypoxia significantly increased IL-6 and IL-10 secretion from MDSCs, thereby increasing MDSC-mediated immunosuppression, decreasing T-cell function, and promoting tumor progression.