Abstract
Objective To test the effectiveness of an integrated collaborative care model for people with depression and long term physical conditions.
Design Cluster randomised controlled trial.
Setting 36 general practices in the north west of England.
Participants 387 patients with a record of diabetes or heart disease, or both, who had depressive symptoms (≥10 on patient health questionaire-9 (PHQ-9)) for at least two weeks. Mean age was 58.5 (SD 11.7). Participants reported a mean of 6.2 (SD 3.0) long term conditions other than diabetes or heart disease; 240 (62%) were men; 360 (90%) completed the trial.
Interventions Collaborative care included patient preference for behavioural activation, cognitive restructuring, graded exposure, and/or lifestyle advice, management of drug treatment, and prevention of relapse. Up to eight sessions of psychological treatment were delivered by specially trained psychological wellbeing practitioners employed by Improving Access to Psychological Therapy services in the English National Health Service; integration of care was enhanced by two treatment sessions delivered jointly with the practice nurse. Usual care was standard clinical practice provided by general practitioners and practice nurses.
Main outcome measures The primary outcome was reduction in symptoms of depression on the self reported symptom checklist-13 depression scale (SCL-D13) at four months after baseline assessment. Secondary outcomes included anxiety symptoms (generalised anxiety disorder 7), self management (health education impact questionnaire), disability (Sheehan disability scale), and global quality of life (WHOQOL-BREF).
Results 19 general practices were randomised to collaborative care and 20 to usual care; three practices withdrew from the trial before patients were recruited. 191 patients were recruited from practices allocated to collaborative care, and 196 from practices allocated to usual care. After adjustment for baseline depression score, mean depressive scores were 0.23 SCL-D13 points lower (95% confidence interval −0.41 to −0.05) in the collaborative care arm, equal to an adjusted standardised effect size of 0.30. Patients in the intervention arm also reported being better self managers, rated their care as more patient centred, and were more satisfied with their care. There were no significant differences between groups in quality of life, disease specific quality of life, self efficacy, disability, and social support.
Conclusions Collaborative care that incorporates brief low intensity psychological therapy delivered in partnership with practice nurses in primary care can reduce depression and improve self management of chronic disease in people with mental and physical multimorbidity. The size of the treatment effects were modest and were less than the prespecified effect but were achieved in a trial run in routine settings with a deprived population with high levels of mental and physical multimorbidity.
Trial registration ISRCTN80309252.
Introduction
Multimorbidity (the presence of two or more long term conditions) is prevalent,1 but the combination of a long term condition and depression is associated with the greatest decrements in quality of life.2 Coexistence of depression is also associated with poorer outcome of physical disorders, increased mortality, and unscheduled care, with considerable cost implications: in the English National Health Service (NHS) the presence of depression increases the cost of care for patients with long term conditions by at least 45% or from £3910 to £5670 (€5181 to €7513; $5892 to $8544) a year.3 4 5 6
In 2005 the World Health Organization proposed that there can be “no health without mental health.”7 And in 2011 the UK coalition government pledged parity of esteem between physical and mental health to reduce inequities between physical and mental health services.8 That policy pledge included an explicit commitment to improve the mental health of people with physical health problems. For people with long term conditions this can potentially be achieved by strengthening primary care to deliver mental health services within the context of management of chronic disease.9
Considerable gains have been made in primary care over recent years to improve access to and quality of depression care, with one promising intervention being “collaborative care,” a complex intervention that involves the use of a non-medical case manager working in conjunction with the patient’s physician (usually their primary care physician), often with the support and supervision of a mental health specialist (normally a psychiatrist or psychologist).10
A recent Cochrane review that included 79 randomised controlled trials concluded that collaborative care is more effective than usual care for both depression and anxiety.11 Only nine trials included in this review, however, tested collaborative care models in which depression care was delivered to populations with recognised long term conditions; only one of these trials was conducted outside the United States, and this tested collaborative care for depression in patients with cancer in a specialist setting.12 In the UK, the National Institute for Health and Care Excellence (NICE) recommends that people with long term conditions with moderate to severe depression and associated functional impairment should be managed with collaborative care, but this guidance is based on a secondary analysis of a weak evidence base.13 Furthermore, there is considerable uncertainty about the effectiveness of integrated collaborative care models for managing depression in patients with multimorbidity in primary care settings that resemble routine care—trials to date have been run in academic primary care with populations without multimorbidity.
We tested the effectiveness of an integrated collaborative care model for people with depression and long term conditions in which interventions were delivered by existing providers and patients had the freedom to choose various psychological treatments and/or drugs for their depression. We targeted patients with depression and diabetes and/or heart disease as exemplar cases. These patients are known to have increased symptoms of depression and high levels of medical comorbidity and thus serve as a test case for the intervention in populations with multimorbidity.
Methods
Study design and participants
The Collaborative Interventions for Circulation and Depression (COINCIDE) trial was conducted by a multidisciplinary team as part of the Greater Manchester Collaboration for Leadership in Applied Health Research and Care (CLAHRC). CLAHRCs are innovative research programmes funded by the UK National Institute for Health Research that support evaluations of interventions most likely to be rapidly adopted in routine clinical practice. COINCIDE was a pragmatic practice level cluster randomised controlled trial with two parallel groups. The trial protocol has been previously published14 and updated.15
This trial was conducted in the English NHS in non-academic primary care general practices. We used a cluster design to avoid contamination of participants in the control group. General practices that held electronic registers of patients with diabetes and/or coronary heart disease were recruited across the north west of England between January and November 2012. Eligible patients were those with a record of diabetes and/or coronary heart disease registered at one of the participating practices who also had depressive symptoms (score ≥10 on the nine item patient health questionnaire (PHQ-9))16 for at least two weeks. Before postal invitations were sent, general practitioners checked the disease registers to exclude ineligible patients (aged under 18, recently deceased, no diabetes or coronary heart disease, or on the palliative care register). We excluded patients with psychosis or type I or type II bipolar disorder; those who were actively suicidal; those in receipt of services for substance misuse; or those in receipt of psychological therapy for depression from a mental health service.
Staff from the National Institute for Health Mental Health Research Network searched electronic records from participating general practices for eligible patients. Patients who met the eligibility criteria received a postal invitation, followed by a reminder letter three weeks later; non-responders to the reminder postal invitation were telephoned. To enhance recruitment of patients of South Asian origin an information flyer about the trial was included in Urdu and Gujarati; the information sheets and consent forms were also translated into Urdu and Gujarati. After the first postal invitation a researcher fluent in Urdu, Hindi, and Punjabi telephoned eligible patients of South Asian origin who did not speak English to provide further information about the study.
After receiving consent forms research staff screened patients for depressive symptoms over the telephone using the PHQ-916 and confirmed eligibility. Patients who scored ≥10 on the PHQ-9 were then visited by a researcher two weeks later and asked to complete a second PHQ-9. If patients also scored ≥10 on the PHQ-9 at the face-to-face visit they were invited to complete baseline assessments.
Randomisation and masking
General practices were randomised as they were recruited by using a central randomisation service provided by the clinical trials unit at the Christie NHS Foundation Trust, Manchester. Allocation of practices (other than the first six, which were allocated 1:1 at random) was by minimisation. This technique ensures “treatment groups that are very closely similar for several variables” even in small samples.17 We used only two practice level variables (index of multiple deprivation18 and list size) and incorporated a random element into this process by which practices were allocated to the trial arm that minimised the imbalance between characteristics with a probability weighting of 0.8.19 The allocation sequence was concealed from general practice staff and from all research staff, except the trial manager, the principal investigator, and the senior investigator with clinical supervisory responsibilities. Patients remained unaware of treatment allocation throughout the telephone screening and the baseline assessment appointments with research staff. Researchers who collected outcome data remained blinded to treatment allocation throughout the course of the trial. Because this trial used face-to-face psychological treatments it was not possible to maintain the blinding of participants beyond baseline or to blind the health professionals delivering the intervention.
Intervention and comparators
Collaborative care
Over three months, participants in the collaborative care arm received up to eight face-to-face sessions of brief psychological therapy delivered by a case manager who were “psychological wellbeing practitioners” employed by Improving Access to Psychological Therapies services in the English NHS. These services offer evidence based psychological treatments for people aged over 16, with no upper age limit, in accordance with stepped care treatment models recommended by NICE.20 Eighteen psychological wellbeing practitioners were involved in delivery of the intervention. Twelve of them were women; their mean age was 35 (SD 9.3); and they had a mean of 3.9 (SD 1.7) years of service.
The first treatment session aimed to be completed within 45 minutes during which the psychological wellbeing practitioner used a structured patient centred interview21 to gather information about the nature of the patient’s key problems, including their experience of the autonomic, behavioural, and cognitive symptoms associated with low mood and anxiety (the ABC model),22 any modifying factors, and the impact of these symptoms, including level of risk. The link between the patient’s mood and management of their diabetes and/or heart disease was explored, and they were introduced to the standardised treatment manual and workbook (see appendix 1 and 2) to help develop a main problem statement and personalised goals. Subsequent sessions lasted for 30-40 minutes. Working with their psychological wellbeing practitioner, participants in the collaborative care arm chose to engage in behavioural activation, graded exposure, cognitive restructuring, and/or lifestyle changes. Treatments took place at the participant’s general practice clinic or at Improving Access to Psychological Therapies business premises.
To better achieve integrated care, a 10 minute collaborative meeting (by telephone or in person) between the patient and the psychological wellbeing practitioner and a practice nurse from the patient’s general practice was scheduled to take place at the end of the second and eighth sessions. Psychological wellbeing practitioners were guided by a manual to run these joint sessions (see appendix 3). These collaborative meetings focused on ensuring that psychological treatments did not complicate management of physical health and patient safety, reviewing patients’ progress with their problem statement and goals, reviewing relevant physical and mental health outcomes (such as depression, anxiety, diet, exercise), and planning future care. Psychological wellbeing practitioners also worked collaboratively with the patient and practice nurse to check that patients adhered to antidepressants as prescribed, dealt with concerns about side effects, and helped to arrange drug reviews with the general practitioner if necessary. In keeping with routine management of patients within Improving Access to Psychological Therapies services, psychological wellbeing practitioners monitored patients’ progress at each session, and delivery of care was structured in accordance with established stepped care protocols.23
Psychological wellbeing practitioners were trained in the COINCIDE collaborative care model over five days by a multidisciplinary team of psychological therapists, an academic general practitioner with special interests in mental health, and a primary care psychiatrist. Cultural competency training was delivered by a psychiatrist with special interests in translation of guided self help materials for people of South Asian origin. The training programme was piloted as part of a separate roll out of collaborative care in another region of the NHS24 and included sessions about diabetes and heart disease along with live and video demonstrations of each treatment session using simulated patients. The final training session focused on strategies for maintaining health and relapse prevention, effective liaison, supervision, and monitoring.
Practice nurses attended a half day workshop, where they met the psychological wellbeing practitioners tasked to work in their general practice and were introduced to the COINCIDE care model with an emphasis on effective liaison and delivering integrated physical and mental health care.
Psychological wellbeing practitioners received one hour of weekly individual supervision by an experienced psychological therapist within their service. New patients, patients at risk of self harm or harming others, poorly responding patients, and patients who did not attend were discussed at weekly supervision, and every case was reviewed during monthly case management supervision.25 Supervisors could consult the trial clinical team about drug management. The COINCIDE team psychiatrist also visited Improving Access to Psychological Therapies teams to discuss how psychological wellbeing practitioners could work flexibly to respond to problems raised by patients—for example, by using the collaborative care approach to manage symptoms of anxiety or to manage depressive symptoms that were not linked to their long term condition. The trial psychiatrist also offered supervisors the option of telephone support.
Usual care
All participants received care as usual from their general practitioner, which could include referral for psychological therapy (including therapy provided by Improving Access to Psychological Therapies) and/or prescription of antidepressants. Psychological wellbeing practitioners who had been trained in the COINCIDE care model were restricted from working with patients allocated to control general practices.
Outcomes
All outcomes were collected at the individual participant level. Participants were followed up initially at six months. After a change in the protocol (see statistical analysis) participants were subsequently followed up at four months. To maximise retention, follow-up assessments were conducted when possible in person, although those who declined a visit were asked to complete the primary outcome measure over the telephone.
The primary outcome was the difference in the mean score on the 13 depression items of the symptom check list-90 (SCL-D13) four months after randomisation.26 The SCL-D13 has 13 items rated from 0-4, and the patients’ overall score on each item is an average of these ratings; higher scores indicate more severe depression. Secondary mental health outcomes were depression and anxiety measured with assessments used routinely in Improving Access to Psychological Therapies (PHQ-9 and generalised anxiety disorder-7 (GAD-7)27), and social support (ENRICHD social support inventory28). Physical health outcomes were global quality of life (WHOQOL-BREF29), disease specific quality of life (diabetes quality of life30 and Seattle angina questionnaire31), and disability (Sheehan disability scale (SDS)32). To assess change in behaviours and perceptions about managing long term conditions we assessed self management (health education impact questionnaire (heiQ)33), self efficacy,34 and illness beliefs (multimorbidity illness perceptions scale35). Process measures collected only at follow-up were patient centredness and care experience (patient assessment of chronic illness care (PACIC)36), and satisfaction with care (client satisfaction questionnaire (CSQ)37). The PACIC questionnaire is widely seen as a valid patient reported assessment of the delivery of high quality care for long term conditions and higher scores confirm hypothesised associations between shared decision making and assessments of quality of care and patient satisfaction.38
All secondary outcomes reported here were prespecified in the trial protocol but not prospectively registered in the trial register because of an administrative error on the part of the trial team.
Sample size
We powered the trial to have 80% power (α=0.05; intraclass correlation coefficient 0.06) to detect a difference between groups on the primary outcome at follow-up equivalent to a standardised effect size of 0.4, for which we required 15 practices per arm and 15 patients per cluster (n=450), allowing for 20% attrition. Our forecast was principally based on the findings of a pilot study of collaborative care in UK primary care that reported an effect size of 0.63 (95% confidence interval 0.08 to 1.07)39 and a subgroup analysis of 10 collaborative care trials that recruited patients with long term conditions (effect size −0.30, 95% confidence interval −0.39 to −0.21).13 We anticipated that the achievable effect size in populations with multimorbidity would be 0.4, which is a little under the average of these two previously published effect sizes.
Average recruitment in the first 11 practices was less than 15 patients per practice. We therefore increased the total number of clusters from 30 to 36, with a target of 10 patients per practice, to give 79% power to detect an effect of 0.4 under the same assumptions. The revised target sample was therefore 360 patients. To ensure that we recruited patients from the additional practices within the lifetime of the trial we reduced follow-up from six to four months. Changes to the protocol were made in agreement with the trial steering committee and published.15
Statistical analysis
We undertook intention to treat analyses for all clinical outcomes, reported in accordance with the Consolidated Standards of Reporting Trials guidelines extension for cluster trials.40 All analyses were undertaken in Stata 13, after a predefined analysis plan was shared with the data monitoring and ethics committee. We analysed outcomes at the end of follow-up using multiple linear regression with robust standard errors to account for the clustering of patients within practices.41 We controlled for baseline values of each outcome, patient age, sex, area deprivation (based on residential postcode), level of limitation of daily activities because of comorbidities,42 and use of antidepressants or antianxiety drugs (currently, previously, never); and at the practice level for the design (minimisation) factors of list size and area deprivation. Multiple imputation was used to estimate missing scale scores and other data values at both baseline and follow-up. It was based on chained equations with all primary and secondary outcomes (at both baseline and follow-up), patient demographics (age, sex, education), diagnoses (diabetes and/or heart disease), activity limitation, practice size, and deprivation. We used 10 multiple imputation sets to provide stability of results.43
We used multiple imputation for the main analysis because this generally provides less biased estimates of effect compared with a complete cases analysis.44 We ran two types of sensitivity analyses. To assess sensitivity of the results to multiple imputation we conducted a second analysis on complete cases that included all the same covariates as the main analysis. A further sensitivity analysis was undertaken, adding in a variable for length of follow-up, to determine if differential follow-up affected inferences. In all analyses we controlled for baseline values and clustering. We also report a further post hoc sensitivity analysis for the primary outcome using a restricted covariate set comprising the baseline outcome values and design factors (list size and area deprivation).
For outcomes with skewness outside the range (−1.5 to 1.5) or kurtosis outside the range (1.5 to 4.5), we used standard errors based on 1000 bootstrapped samples to derive confidence intervals and P values. To ease interpretation and to allow comparison with published studies, we estimated standard effect sizes as the difference in follow-up means divided by the pooled baseline standard deviation for all participants.
Results
Participant flow and retention
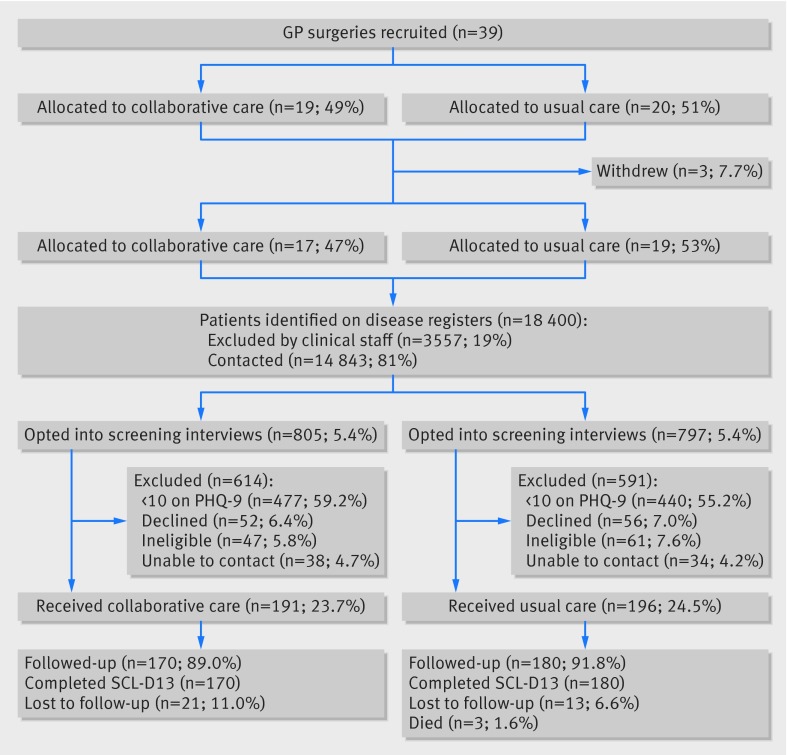
We invited 459 practices and allocated 39 of them, 19 to collaborative care and 20 to usual care; three withdrew before patients were invited to take part. Table 1 shows characteristics of included clusters. Practices in the two arms were similar in all characteristics. Mental Health Research Network staff ran the first electronic searches for patients on 10 April 2012 and sent the last mail out on 18 March 2013. The first participant was recruited on 18 May 2012, and the last participant was recruited on 31 May 2013. A mean of 10.7 (SD 6.2, range 2-22) participants were recruited from each of the 36 practices. We identified 387 patients with depression and heart disease and/or diabetes and invited them to a baseline assessment (figure). Follow-up data on 350 (90%) participants were collected between 18 November 2012 and 4 October 2013.
Table 1.
Cluster (general practices) characteristics of practices included in study of integrated primary care for patients with mental and physical multimorbidity. Figures are numbers or numbers (percentage) of practices unless specified otherwise
| Intervention (n=17) | Control (n=19) | |
|---|---|---|
| Area: | ||
| Merseyside | 7 | 2 |
| Greater Manchester | 8 | 11 |
| East Lancashire | 2 | 6 |
| List size: | ||
| Small <4500 | 9 (53) | 8 (42) |
| Medium 4500-7500 | 4 (23) | 5 (26) |
| Large >7500 | 4 (23) | 6 (31) |
| Deprivation: | ||
| Affluent | 6 (35) | 6 (31) |
| Moderately deprived | 5 (29) | 7 (37) |
| Heavily deprived | 6 (35) | 6 (31) |
| Patients on quality and outcomes framework disease registers: | ||
| Coronary heart disease | 3521 | 4694 |
| Diabetes | 4367 | 5818 |
| Mean participants/practice (SD) with diabetes | 6.2 (3.5) | 5.6 (3.4) |
| Mean participants/practice (SD) with coronary heart disease | 3.7 (2.1) | 3.7 (2.6) |
| Mean participants/practice (SD) with both | 2.4 (1.1) | 1.7 (1.6) |
CONSORT flow diagram of recruitment of general practices and patients in study of integrated primary care for patients with mental and physical multimorbidity. At four months, 106 (62.4%) participants assigned to collaborative care and 161 (89.4%) assigned to usual care were followed up. At six months 64 (37.6%) participants assigned to collaborative care and 19 (10.6%) assigned to usual care were followed up
Baseline characteristics of participants
Three quarters of participants (295/387; 76%) were recruited from practices from moderately and heavily deprived areas, over half of whom (125; 54%) came from areas ranked as highly deprived (index of multiple deprivation score ≥30). Most (245; 64%) met criteria for moderately severe or severe depression, and 290 (75%) met criteria for anxiety. Participants had a mean of 6.2 (SD 3.0) medical conditions in addition to either diabetes or coronary heart disease; 15% of participants had a diagnosis of both diabetes and coronary heart disease. Just under two thirds (62%) of participants were men, and the mean age was 58.5 (SD 11.7); only 96 (25%) participants were in paid employment. Half of participants were prescribed antidepressants or antianxiety drugs at baseline (table 2). Patients in the two arms were similar in all respects, except that a higher percentage of usual care arm patients were in large practices (106 (54%) v 70 (37%)).
Table 2.
Baseline characteristics of participants in study of integrated primary care for patients with mental and physical multimorbidity. Figures are numbers (percentage) of patients
| Intervention (n=191) | Usual care (n=196) | |
|---|---|---|
| Practice deprivation*: | ||
| Affluent | 49 (26) | 43 (22) |
| Moderately deprived | 80 (42) | 90 (46) |
| Heavily deprived | 62 (32) | 63 (32) |
| Practice size*: | ||
| Small (<4500) | 60 (31) | 49 (25) |
| Medium (4500-7500) | 61 (32) | 41 (21) |
| Large (>7500) | 70 (37) | 106 (54) |
| Mean (SD) age (years) | 57.9 (12.0) | 59.2 (11.4) |
| Men | 113 (59) | 127 (65) |
| White | 164 (86) | 167 (85) |
| Mean (SD) deprivation (IMD score) | 36.6 (21.3) | 34.4 (18.5) |
| In owner occupied accommodation | 115 (60) | 122 (62) |
| In paid employment (full or part time) | 49 (26) | 47 (24) |
| Index medical condition by quality and outcomes framework register: | ||
| Diabetes | 106 (55) | 101 (51) |
| Coronary heart disease (CHD) | 56 (29) | 66 (34) |
| Both | 29 (15) | 29 (15) |
| Mean (SD) No of long term conditions (other than diabetes or CHD) | 6.0 (3.2) | 6.5 (3.0) |
| Mean (SD) SCL-D-13 total (0-4) | 2.36 (0.70) | 2.33 (0.82) |
| Mean (SD) PHQ-9 total (0-27) | 16.4 (4.2) | 16.5 (4.1) |
| PHQ-9 score by symptom severity: | ||
| 10-14 (moderate) | 68 (36) | 73 (37) |
| 15-19 (moderate to severe) | 79 (41) | 76 (39) |
| 20-27 (severe) | 44 (23) | 47 (24) |
| Mean (SD) GAD-7 total (0-21) | 12.3 (5.1) | 11.9 (5.3) |
| GAD-7 score by symptom severity: | ||
| 0-4 (mild) | 15 (7.9) | 12 (6.1) |
| 5-9 (moderate) | 42 (22.0) | 61 (31.1) |
| 10-14 (moderate to severe) | 72 (38.5) | 56 (28.6) |
| 15-21 (severe) | 62 (32.5) | 67 (34.2) |
| Prescribed antidepressants | 59 (31) | 73 (37) |
| Prescribed antianxiety drugs | 32 (17) | 30 (15) |
GAD=generalised anxiety disorder assessment; IMD=index of multiple deprivation; PHQ=patient health questionnaire; SCL-D13=symptom checklist depression scale.
*Minimisation variables.
Delivery of the intervention
Psychological wellbeing practitioners each treated a mean of nine patients (SD 6.3, range 1-21). Patients received a mean of 4.4 sessions (SD 3.3, range 0-14); 24 (12.6%) patients received eight treatment sessions and 67 (35%) received at least six sessions. Twenty two (11%) participants in the collaborative care arm did not attend any treatment sessions and disengaged immediately after referral; 42 (22%) participants did not attend any scheduled treatment session, and 30 (16%) did not attend two or more scheduled treatment sessions. Participants who disengaged immediately from therapy or did not attend scheduled therapy sessions were still followed-up unless they withdrew from the trial. Fifty (26%) participants attended one joint integrated care session; 46 (24%) attended two sessions; 95 (50%) did not attend any session. The mean length of mental health treatment sessions was 27 (SD 29.7) minutes, and the mean length of integrated care sessions was 19.7 (SD 11.9) minutes. There was a small but non-significant increase in use of antidepressants during the trial (6% increase in the collaborative care group, 7% in the usual care group).
Primary outcome
We collected primary outcome data for 350 participants. At the end of follow-up depression scores (on the SCL-D13) declined in both groups, but the decline was greater in the collaborative care arm. The mean depression score at follow-up was 0.23 points lower (95% confidence interval −0.41 to −0.05) in participants who received collaborative care compared with those who received usual care, after adjustment for baseline depression and minimisation variables (table 3). The samples sizes, group means, and standard deviations in table 3 are based on participants who provided data at follow-up, but the estimated difference in means and 95% confidence intervals are taken from the intention to treat analysis (that is, using multiple imputation for missing baseline and follow-up data points). The intracluster coefficient for the primary outcome was 0.03 (0 to 0.10). The difference found between the groups is equal to a standardised mean difference of −0.30 (−0.54 to −0.07), with baseline standard deviation for SCL-D13 (pooled).
Table 3.
Intention to treat analyses of primary and secondary outcomes at four month follow-up in study of integrated primary care for patients with mental and physical multimorbidity
| Intervention | Usual care | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of patients | Mean (SD) | No of patients | Mean (SD) | Adjusted difference in means (95% CI)*, P value | Effect size (95% CI)† | Intracluster coefficient | |||
| Primary outcome | |||||||||
| SCL-D-13 (0-4) | 170 | 1.76 (0.9) | 180 | 2.02 (0.9) | −0.23 (−0.41.to −0.05), 0.01 | −0.30 (−0.54 to −0.07) | 0.03 | ||
| Secondary outcomes | |||||||||
| PHQ-9 (0-27) | 157 | 11.3 (6.5) | 168 | 13.1 (6.5) | −1.2 (−2.37 to −0.03), 0.04 | −0.29 (−0.57 to −0.01) | 0.02 | ||
| GAD-7 (0-21) | 157 | 8.2 (5.8) | 168 | 9.7 (5.9) | −1.45 (−2.45 to −0.56), 0.006 | −0.28 (−0.47 to -0.09) | 0.00 | ||
| ESSI (0-34) | 155 | 3.29 (1.1) | 165 | 3.4 (1.0) | 0.01 (−0.19 to 0.22), 0.91 | 0.01 (−0.18 to 0.20) | 0.07 | ||
| Health education impact questionnaire (heiQ): | |||||||||
| Positive engagement | 155 | 2.48 (1.3) | 164 | 2.32 (1.4) | 0.20 (−0.07 to 0.48), 0.14 | 0.16 (−0.06 to 0.39) | 0.02 | ||
| Health directed behaviour | 155 | 1.92 (1.8) | 164 | 1.65 (1.5) | 0.06 (−0.28 to 0.39), 0.72 | 0.04 (−0.18 to 0.26) | 0.05 | ||
| Skill acquisition | 153 | 2.76 (1.1) | 163 | 2.52 (1.2) | 0.26 (0.02 to 0.50), 0.04 | 0.25 (0.02 to 0.48) | 0.04 | ||
| Constructive attitudes | 155 | 2.83 (1.2) | 165 | 2.64 (1.3) | 0.31 (0.07 to 0.55), 0.01 | 0.25 (0.05 to 0.44) | 0.02 | ||
| Self monitoring | 155 | 3.65 (0.7) | 165 | 3.32 (1.0) | 0.31 (0.01 to 0.52),‡ 0.004 | 0.36 (0.11 to 0.60) | 0.08 | ||
| Health service navigation | 155 | 2.67 (1.0) | 165 | 3.35 (1.2) | 0.28 (0.03 to 0.53), 0.03 | 0.27 (0.03 to 0.52) | 0.06 | ||
| Social integration | 155 | 3.03 (1.3) | 165 | 3.0 (1.4) | −0.01 (−0.32 to 0.31), 0.10 | −0.01 (−0.25 to 0.24) | 0.08 | ||
| Emotional wellbeing | 155 | 2.65 (1.3) | 165 | 3.09 (1.2) | −0.35 (−0.61 to −0.09), 0.01 | −0.32 (−0.55 to −0.08) | 0.05 | ||
| MULTIPleS | 151 | 2.1 (0.9) | 165 | 2.28 (0.9) | −0.14 (−0.34 to 0.06), 0.17 | −0.18 (−0.43 to 0.08) | 0.09 | ||
| SDS (0-10) | 153 | 5.73 (2.8) | 163 | 5.83 (2.8) | −0.27 (−0.75 to 0.20), 0.24 | −0.11 (−0.29 to 0.08) | 0.00 | ||
| SEQ | 155 | 5.72 (1.9) | 166 | 5.54 (1.9) | 0.21 (−0.15 to 0.58), 0.24 | 0.13 (−0.09 to 0.34) | 0.02 | ||
| WHOQOL-BREF | 152 | 2.99 (0.6) | 167 | 2.91 (0.6) | 0.07 (−0.05 to 0.19), 0.26 | 0.13 (−0.10 to 0.36) | 0.02 | ||
ESSI=Enrichd social support inventory; GAD=generalised anxiety disorder assessment; MULTIPleS=multimorbidity illness perception scales; SCL=symptom check list; SEQ=self efficacy questionnaire; SDS=Sheehan disability scale; SCL-D-13=symptom checklist depression scale; WHOQOL-BREF=WHO quality of life measure.
*Analyses with multiple imputation for missing baseline and follow-up data points adjusted for baseline measures of outcome and minimisation variables (practice deprivation and practice size).
†Effect size based on pooled SD of baseline measures.
‡Estimated with bootstrapping.
Secondary outcomes
The benefits of the intervention extended to some but not all secondary outcomes. Participants in the collaborative care arm reported fewer symptoms of anxiety at follow-up compared with those in the usual care arm, equating to a reduction of 1.45 points (95% confidence interval −2.45 to −0.56) on the generalised anxiety disorder scale (GAD-7), equivalent to a standardised mean difference of −0.28 (−0.47 to −0.09). Core aspects of self management on five of the eight domains of the health education impact questionnaire (heiQ) significantly improved in patients in the collaborative care arm (table 3).
We observed no significant differences between groups for disability, self efficacy, illness perceptions, and global quality of life (table 3) or for disease specific quality of life (table 4).
Table 4.
Intention to treat analyses of physical disease quality of life at follow-up in study of integrated primary care for patients with mental and physical multimorbidity
| Intervention | Usual care | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of patients | Mean (SD) | No of patients | Mean (SD) | Adjusted difference in means (95% CI)*, P value | Effect size† | Intracluster coefficient | |||
| Diabetes quality of life questionnaire | |||||||||
| Satisfaction with treatment | 111 | 54.6 (18.5) | 118 | 53.7 (17.9) | −0.72 (−4.18 to 2.74), 0.67 | −0.04 | 0.03 | ||
| Impact of treatment | 112 | 65.3 (15.6) | 120 | 63.9 (16.5) | −0.99 (−3.91 to 1.94), 0.50 | −0.07 | 0.07 | ||
| Diabetes worries | 26 | 70.2 (23.6) | 31 | 76.1 (28.8) | 3.52 (−15.13 to 22.17), 0.61 | 0.13 | 0.04 | ||
| Social/vocational worries | 97 | 66.0 (23.1) | 111 | 63.7 (26.8) | 0.52 (−5.40 to 6.43), 0.86 | 0.02 | 0.11 | ||
| Seattle angina questionnaire | |||||||||
| Physical limitation | 78 | 42.3 (28.5) | 88 | 41.2 (28.9) | 2.02 (−3.52 to 7.56), 0.46 | 0.08 | 0.00 | ||
| Angina stability | 77 | 52.6 (24.9) | 85 | 47.9 (27.1) | 5.32 (−2.15 to 12.80), 0.16 | 0.21 | 0.00 | ||
| Angina frequency | 81 | 70.5 (28.4) | 83 | 68.0 (26.7) | 2.30 (−3.05 to 7.64), 0.39 | 0.09 | 0.08 | ||
| Treatment satisfaction | 79 | 79.2 (17.3) | 81 | 71.9 (24.5) | 5.08 (−2.06 to 12.21), 0.16 | 0.27 | 0.05 | ||
| Quality of life | 79 | 51.6 (24.3) | 82 | 46.0 (27.5) | 5.50 (−1.47 to 12.47), 0.18 | 0.24 | 0.03 | ||
*Analyses with multiple imputation for missing baseline and follow-up data points adjusted for baseline measures of outcome and minimisation variables (practice deprivation and practice size).
†Effect size based on pooled SD of baseline measures.
Process data showed that participants in the collaborative care arm rated the delivery and experience of care as more patient centred. Patients’ scores on all five subscales of the patient assessment of chronic illness care (PACIC) were higher in the collaborative care arm than in the usual care arm, as was the total score on this scale (2.37 (SD 1.0) v 1.98 (SD 0.9)), equivalent to an unadjusted standardised mean difference of 0.39. Based on total scores on the client satisfaction questionnaire (CSQ) participants in the collaborative care arm were also more satisfied with their care at follow-up compared with usual care (2.90 (SD 0.6) v 2.62 (SD 0.6)), equivalent to an unadjusted standardised mean difference of 0.53 (table 5).
Table 5.
Mean difference and effect sizes for process of care measures at follow-up in study of integrated primary care for patients with mental and physical multimorbidity
| Intervention | Usual care | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| No of patients | Mean (SD) | No of patients | Mean (SD) | Difference in means (95% CI) | Effect size* | |||
| Patient assessment of chronic illness care (PACIC) | ||||||||
| Patient activation | 153 | 2.50 (1.4) | 159 | 2.08 (1.2) | 0.42 (0.13 to 0.71) | 0.32 | ||
| Delivery system design/decision support | 153 | 2.71 (1.2) | 159 | 2.29 (1.1) | 0.42 (0.17 to 0.67) | 0.36 | ||
| Goal setting | 153 | 2.18 (1.2) | 158 | 1.77 (1.0) | 0.41 (0.16 to 0.66) | 0.37 | ||
| Problem solving/contextual counselling | 154 | 2.65 (1.2) | 158 | 2.28 (1.2) | 0.37 (0.10 to 0.64) | 0.31 | ||
| Follow-up/coordination | 153 | 2.00 (0.9) | 158 | 1.77 (1.0) | 0.23 (−0.08 to 0.54) | 0.16 | ||
| Total score | 155 | 2.37 (1.1) | 163 | 1.98 (1.0) | 0.39 (0.16 to 0.62) | 0.37 | ||
| Client satisfaction questionnaire (CSQ) | ||||||||
| Quality of service | 155 | 2.90 (0.9) | 158 | 2.51 (1.0) | 0.39 (0.18 to 0.60) | 0.41 | ||
| Kind of service you wanted | 155 | 2.97 (0.8) | 159 | 2.63 (0.9) | 0.34 (0.15 to 0.53) | 0.40 | ||
| Satisfied with help | 152 | 2.51 (1.0) | 156 | 2.42 (1.0) | 0.09 (−0.13 to 0.31) | 0.09 | ||
| Services helped | 152 | 3.11 (0.8) | 158 | 2.68 (0.9) | 0.43 (0.24 to 0.62) | 0.50 | ||
| Satisfied overall | 152 | 2.95 (0.9) | 154 | 2.48 (0.8) | 0.47 (0.28 to 0.66) | 0.53 | ||
| Come back to service | 153 | 2.96 (0.8) | 157 | 2.48 (0.8) | 0.48 (0.30 to 0.60) | 0.60 | ||
| Total score | 156 | 2.90 (0.6) | 160 | 2.58 (0.6) | 0.32 (0.19 to 0.45) | 0.53 | ||
*Effect size based on pooled SD for collaborative care and usual care.
Missing data and sensitivity analyses
Telephone interviews were used to collect primary outcome data only among patients who declined a face to face follow-up assessment; 37 (9.6%) patients had missing data for the primary outcome. There was a higher percentage of missing data for secondary outcomes, but no scale had more than 20% missing data. We used multiple imputation for the main analysis to take account of this. Sensitivity analysis with a complete cases approach returned the same pattern of significant results, with the exception of self management behaviours measured on the health education impact questionnaire, with significant differences for two (instead of five) domains: self monitoring and health service navigation (table 6). Departure from normality occurred only for self monitoring and insight on the health education impact questionnaire (heiQ) scale, when the central estimate and 95% confidence interval for both the multiple imputation and complete cases analyses were estimated via bootstrapping. A second sensitivity analysis, adjustment for length of follow-up (six v four months) had no important effect on the inferences made in the main analysis.
Table 6.
Sensitivity analyses of primary and secondary outcomes at four month follow-up for complete cases in study of integrated primary care for patients with mental and physical multimorbidity
| Intervention | Usual care | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of patients | Mean (SD) | No of patients | Mean (SD) | Adjusted difference in means (95% CI)*, P value | Effect size (95% CI)† | Intracluster coefficient | |||
| Primary outcome | |||||||||
| SCL-D-13 (0-4) | 170 | 1.76 (0.9) | 180 | 2.02 (0.9) | −0.24 (−0.38 to −0.11), 0.001 | −0.32 (−0.50 to −0.14) | 0.03 | ||
| Secondary outcomes | |||||||||
| PHQ-9 (0-27) | 157 | 11.3 (6.5) | 168 | 13.1 (6.5) | −1.20 (−2.37 to −0.04), 0.04 | −0.29 (−0.57 to −0.01) | 0.02 | ||
| GAD-7 (0-21) | 157 | 8.2 (5.8) | 168 | 9.7 (5.9) | −1.61 (−2.57 to −0.66), 0.002 | −0.31 (−0.49 to −0.09) | 0.00 | ||
| ESSI (0-34) | 155 | 3.29 (1.1) | 165 | 3.4 (1.0) | 0.00 (−0.20 to 0.21), 0.96 | 0.00 (−0.19 to 0.19) | 0.07 | ||
| Health education impact questionnaire (heiQ): | |||||||||
| Positive engagement | 155 | 2.48 (1.3) | 164 | 2.32 (1.4) | 0.12 (−0.11 to 0.36), 0.29 | 0.10 (−0.09 to 0.29) | 0.02 | ||
| Health directed behaviour | 155 | 1.92 (1.8) | 164 | 1.65 (1.5) | 0.04 (−0.30 to 0.37), 0.83 | 0.02 (−0.20 to 0.23) | 0.05 | ||
| Skill acquisition | 153 | 2.76 (1.1) | 163 | 2.52 (1.2) | 0.20 (−0.08 to 0.49), 0.16 | 0.19 (−0.08 to 0.47) | 0.04 | ||
| Constructive attitudes | 155 | 2.83 (1.2) | 165 | 2.64 (1.3) | 0.20 (−0.04 to 0.45), 0.10 | 0.17 (−0.03 to 0.04) | 0.02 | ||
| Self monitoring | 155 | 3.65 (0.7) | 165 | 3.32 (1.0) | 0.29 (0.08 to 0.49),‡ 0.007 | 0.33 (0.05 to 0.61) | 0.08 | ||
| Health service navigation | 155 | 2.67 (1.0) | 165 | 3.35 (1.2) | 0.29 (0.07 to 0.49), 0.01 | 0.28 (0.07 to 0.49) | 0.06 | ||
| Social integration | 155 | 3.03 (1.3) | 165 | 3.0 (1.4) | 0.00 (−0.30 to 0.29), 0.97 | 0.00 (−0.23 to 0.23) | 0.08 | ||
| Emotional wellbeing | 155 | 2.65 (1.3) | 165 | 3.09 (1.2) | 0.27 (−0.54 to 0.01), 0.06 | −0.24 (−0.48 to 0.01) | 0.05 | ||
| MULTIPleS | 151 | 2.1 (0.9) | 165 | 2.28 (0.9) | −0.10 (−0.28 to 0.07), 0.24 | −0.13 (−0.36 to 0.09) | 0.09 | ||
| SDS (0-10) | 153 | 54.3 (30.5) | 163 | 55.3 (29.7) | −0.10 (−0.55 to 0.34), 0.67 | −0.04 (−0.22 to 0.14) | 0.00 | ||
| SEQ | 155 | 5.72 (1.9) | 166 | 5.54 (1.9) | 0.08 (−0.30 to 0.50), 0.66 | 0.05 (−0.17 to 0.27) | 0.02 | ||
| WHOQOL-BREF | 152 | 2.99 (0.6) | 167 | 2.91 (0.6) | 0.05 (−0.07 to 0.17), 0.38 | 0.10 (−0.13 to 0.32) | 0.02 | ||
ESSI=Enrichd social support inventory; GAD=generalised anxiety disorder assessment; MULTIPleS=multimorbidity illness perception scales; SCL-D-13=symptom checklist depression scale; SEQ=self efficacy questionnaire; SDS=Sheehan disability scale; WHOQOL-BREF=WHO quality of life measure.
*Adjusted for baseline measures of outcome and minimisation variables (practice deprivation and practice size).
†Effect size based on pooled SD of baseline measure.
‡Estimated via bootstrapping.
The post hoc additional analysis of the self reported symptom checklist-13 depression scale (SCL-D13) with a restricted covariate set made only a small difference to the estimate of effect: 0.26 points (95% confidence interval −0.42 to −0.08), equivalent to a standardised mean difference of −0.34 (−0.56 to −0.11).
We received notification of four deaths unrelated to delivery of the intervention. Three deaths occurred in control practices; one death occurred in an intervention practice but after outcome data had been collected at follow up.
Discussion
Collaborative care that integrated brief psychological interventions within the context of routine primary care management of long term conditions reduced depressive symptoms more than usual care in patients with multimorbidity. Participants in the collaborative care arm also reported significantly less anxiety at follow-up. The observed treatment effect size (0.3) in our trial was modest and lower than the prespecified effect (0.4) but similar to that achieved in a previous UK study of collaborative care in adults without multimorbidity45 and comparable with the overall effect size for collaborative care of 0.29 reported in a Cochrane review in 2012.11 While these treatment effects for depression are modest, they were achieved in the context of a pragmatic trial that included participants with considerable levels of mental and physical multimorbidity and high levels of socioeconomic deprivation. In this sense our findings appeal to the need for research about how to potentially integrate mental and physical healthcare among disadvantaged populations with broader multimorbidity from deprived areas.46 47
The benefits of collaborative care extended beyond reductions in depressive and anxiety symptoms, with patients rating themselves as better self managers. Self management is increasingly seen as critical to the delivery of effective and efficient care for long term conditions, but achieving effective self management is a considerable challenge in patients with multimorbidity. This in part stems from system level barriers because organisation of primary care is often poorly matched to the needs and experiences of patients living with multimorbidity.48 There are two possible mechanisms that might explain why patients in our trial thought they could manage their symptoms more effectively: they were less depressed and thus more confident and engaged in their care, or the delivery of their care improved—for example, through provision of joint therapy sessions—and this was highlighted by higher ratings for patient centredness in the collaborative care arm.
Improvements in self management behaviours are hypothesised to be antecedents to improvements in physical health,33 but we did not test this association in this trial. In the US the TEAMcare trial, which was a high intensity intervention for depression and diabetes or heart disease delivered in academic settings over 12 months, observed significant improvements in social role disability and global quality of life.49 Large improvements in functional outcomes among depressed patients have typically been achieved in trials that used more intensive interventions.50 Our trial offered a more limited less intensive intervention and did not see comparable improvements in disability or quality of life. Similarly, while the ENRICHD trial showed that high intensity therapies such as cognitive behavioural therapy can improve social support in patients with depression and heart disease, we did not observe such improvements.51 It is not clear what ingredients of collaborative care are responsible for improvements in functional and social support outcomes, but self efficacy has been proposed as a mediator that might strengthen depression interventions. TEAMcare did observe improvements in self efficacy whereas we did not.52
Strengths and limitations
This was a large pragmatic trial conducted across a wide geographical area in the north west of England that included considerable socioeconomic deprivation and high densities of ethnic minorities. We recruited participants by systematically searching disease registers and screening for depression. There is evidence that this method of recruitment can identify patients who have additional capacity to benefit from collaborative care.53 There was minimal attrition and no evidence that missing data or differential follow-up affected the results. Our findings, however, should be interpreted in the context of several limitations. Firstly, we could not recruit and assess participants before practices were randomised, leading to concerns that allocations might not have been concealed from practice staff and participants. Because participants were recruited in a serial fashion from each practice, however, it was not practicable to postpone allocation to treatment groups until all participants had been recruited. To aid allocation concealment, however, we used non-clinical staff to identify potential participants at each practice. Secondly, we were able to assess only the short term effectiveness of collaborative care, and we do not know if the positive effects persist beyond four months. Our first goal, however, was to test the feasibility and effectiveness of collaborative care in routine settings among under-served patients with long term conditions, and it is encouraging that we found positive effects for depression at four months. Additionally, four months has been used as the primary end point to test the effectiveness of collaborative care,45 and we would subscribe to the view this is the earliest time point at which we might expect to see a treatment effect for psychological therapies based on a behavioural modification framework.54 Furthermore, while depression is for some people a long term problem, short term benefits are still likely to be important outcomes for patients. Thirdly, despite the use of self reported questionnaires and masking of research staff to allocation, all outcome data were collected face to face at follow-up, and researchers might have been made aware of treatment allocations, leading to assessment bias. We did not formally test for this type of bias, which could have been done by interviewing researchers to determine whether they knew to which treatment group participants had been allocated. Fourthly, we collected only self reported data on the use of antidepressants. Future trials of this kind should make efforts to also collect data on changes to the type and dose of antidepressant so as to be able to judge whether patients who do not initially respond to treatment are more likely to be switched to an alternative antidepressant in the context of collaborative care. Fifthly, we did not collect objective measures of physical functioning and were thus unable to assess the impact of collaborative care on both physical and mental health. Unlike the TEAMcare55 trial, which used treat-to-target protocols for diabetes and heart disease, however, our intervention focused primarily on treating depression but also imparted skills and empowered patients to more effectively self manage their long term conditions. Finally, because we notified general practitioners in both arms that participants met criteria for depression the effect of the intervention might have been reduced. This is unlikely given that screening for depression alone does not lead to changes in the management of depression.56
Implications for practice, research, and policy
Our trial tested whether mental health workers with limited experience of collaborative care can be trained to routinely work alongside primary care nurses to deliver a simple and integrated care model for patients with high levels of mental and physical multimorbidity. The novel aspects of our trial have broad relevance for implementation of integrated care for mental-physical multimorbidity in routine settings. In the UK, the CADET trial has showed that the benefits of collaborative care for depression translate to settings outside the US.45 CADET did not specifically recruit patients with long term conditions, however, and the relevance of findings in that trial for populations with multimorbidity are not known. Landmark US trials of collaborative care for patients with depression and long term conditions similarly did not include patients with multimorbidity, and participants in these trials were less depressed at baseline than those in COINCIDE.55 57 This is also true of the TrueBlue58 trial in Australia, which tested nurse led collaborative care for depression in patients with diabetes or heart disease. Half the patients in that trial had mild (sub-threshold) depression and significant treatment effects were reported only in a subgroup with moderate to severe depression. Such subgroup analyses are controversial.59 Additionally, as with Teamcare, the course of treatment in TrueBlue lasted 12 months, reducing the relevance of these findings in primary care settings where the average course of brief psychological interventions is typically six sessions.60 By contrast, patients in COINCIDE were more deprived and more depressed and had higher levels of multimorbidity than comparable collaborative care trials. This is true even of the IMPACT trial,61 which tested collaborative care for depression in older adults, lending further support to the finding that younger adults with depression from deprived areas have higher rates of multimorbidity than older populations.1
Compared with previous collaborative care trials, COINCIDE tested a broader range of psychological treatments (that is, behavioural activation, cognitive restructuring, graded exposure, and lifestyle approaches, with the option to stay or start taking antidepressants), and they were tailored to meet the needs of patients with long term conditions, thus enhancing the integration of physical and mental healthcare. Half the patients in COINCIDE attended a joint meeting with their practice nurse and psychological therapist, and this level of integration between mental and physical healthcare providers in primary care is unprecedented. It could equally be argued, however, that the level of collaboration between psychological wellbeing practitioners and nurses was minimal, suggesting that the positive effects for depression were attributable to the presence of the psychological wellbeing practitioner and not the collaborative framework. There is good evidence that collaborative care that includes psychological therapy with or without drugs is more effective than collaborative care that includes only drugs, and our trial would support this finding.53 We do not, however, have definitive evidence about the mechanisms that led to improved depression outcomes in our trial, and future research is needed that can model the effects of both intervention and individual patient level moderators on treatment outcomes.
Previous collaborative care trials for depression and long term conditions have also relied on input from academic supervisors, whereas in COINCIDE therapists were supervised by existing providers. In this sense the COINCIDE trial is a test case of how a brief and integrated collaborative care model can be rolled out at a pace and scale within the context of chronic disease management by using existing providers and without greatly altering arrangements for clinical supervision. Proof of this is evidenced by the uptake of the COINCIDE care model among Improving Access to Psychological Therapies services in England as part of a phased National Institute for Health Research funded roll out and evaluation of collaborative care for people with long term conditions and common mental health problems.62 Following a commitment to extend Improving Access to Psychological Therapies to meet the needs of patients with long term conditions, this roll out involves the COINCIDE team training the psychological wellbeing practitioner workforce to work collaboratively in primary care to deliver low intensity psychological treatments to patients with a wide range of long term conditions. Long term implementation is underpinned by delivery of a “train the trainer” programme, in which clinical champions from participating Improving Access to Psychological Therapies services will take on responsibility for future training of psychological wellbeing practitioners to work with the COINCIDE care model. This initiative bridges the gap from evidence to practice through effective partnership working between research leaders, service providers, and commissioners to meet the needs of complex patients with multimorbidity.
Conclusions
This trial answers the call to better understand how to integrate mental healthcare in general healthcare through developing innovative care models and strengthening close links to specialist services.63 For the first time we have shown that patients with high levels of mental and physical multimorbidity can gain modest but important benefits from brief low intensity psychological interventions delivered in partnership with practice nurses, rather than waiting to be stepped up to more intensive and less available treatments, as currently recommended in England by the NICE.20 As has been shown in cancer settings,64 patients with medical comorbidities benefit more if their depression is proactively managed with an integrated collaborative care approach. It is imperative for the health and wellbeing of people with mental and physical multimorbidity that future research focuses on how best to translate such integrated care models into routine primary care.
What is already known on this topic
Mental and physical multimorbidity is highly prevalent among primary care populations, but few interventions exist to improve mental health outcomes in this group
Collaborative care can improve depression in people with long term conditions, but most studies have been conducted in the United States, have recruited highly selected groups of patients without multimorbidity, and have used elite academic teams to deliver and supervise interventions
There is emerging evidence that collaborative care can translate to non-US settings, but there is uncertainty if these benefits are realisable in more routine settings and among patients with multimorbidity
What this study adds
Collaborative care that integrates brief low intensity psychological treatment within primary care can reduce depression and improve self management in the short term in people with multimorbidity, but the size of effects is modest
Mental health providers and practice nurses with limited experience of collaborative care can be trained to deliver high quality and patient centred integrated healthcare for people with mental and physical multimorbidity
The COINCIDE trial offers a template for how integrated collaborative care can be potentially implemented within the context of routine chronic disease management with only minimal changes to the organisation of primary care
Contributors: PC, KL, CD, PB, CC-G, AC, CG, CJG, CB, KR, IA, WW, MH, and LG were responsible for drafting and revising the original protocol. PC was the chief investigator and had overall responsibility for management of the trial. KL, CC-G, LG, CB, WW, and LG delivered the training to practice nurses, psychological wellbeing practitioners, and clinical supervisors. LG provided additional clinical supervision and risk assessment training. CG, CJG, KA, and IA collected the data. DM wrote the analysis plan and cleaned and analysed the data under supervision from MH and DR. PC wrote the first draft of the report and revised subsequent draft. All authors contributed to and approved the final report. PC is guarantor.
Funding: This trial was funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care for Greater Manchester. The views expressed in this article are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: all authors had financial support from NIHR for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study was approved by the national research ethics service committee North West-Preston (NRES/11/NW/0742); research governance approvals were granted by participating primary care trusts and informed consent was given by all patients.
Data sharing: Patient level data is available from the corresponding author. Consent for data sharing was not obtained but the presented data are anonymised and risk of identification is low.
Transparency declaration: PC affirms that the manuscript is an honest, accurate, and transparent account of the research findings and no important aspects of the study have been omitted.
Cite this as: BMJ 2015;350:h638
Web Extra. Extra material supplied by the author
Appendix 1: COINCIDE Patient Manual
Appendix 2: COINCIDE Workbook
Appendix 3: COINCIDE PWP Manual
References
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. [DOI] [PubMed] [Google Scholar]
- 2.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370:851-8. [DOI] [PubMed] [Google Scholar]
- 3.Naylor C, Parsonage M, McDaid D, Knapp M, Fossey M, Galea A. Long-term conditions and mental health. The cost of co-morbidities. King’s Fund and Centre for Mental Health, 2012.
- 4.Carney RM, Freedland KE, Sheps DS. Depression is a risk factor for mortality in coronary heart disease. Psychosom Med 2004;66:799-801. [DOI] [PubMed] [Google Scholar]
- 5.Hjerl K, Andersen EW, Keiding N, Mouridsen HT, Mortensen PB, Jorgensen T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics 2003;44:24-30. [DOI] [PubMed] [Google Scholar]
- 6.Dickens C, Katon W, Blakemore A, Khara A, McGowan L, Tomenson B, et al. Does depression predict the use of urgent and unscheduled care by people with long term conditions? A systematic review with meta-analysis. J Psychosomatic Res 2012;73:334-42. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Mental health: facing the challenges, building solutions. Report from the WHO European Ministerial Conference. WHO, 2005. [PubMed]
- 8.Department of Health. No health without mental health. A cross-government mental health outcomes strategy for people of all ages. Stationery Office, 2011.
- 9.Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. No health without mental health. Lancet 2007;370:859-77. [DOI] [PubMed] [Google Scholar]
- 10.Gunn J, Diggens J, Hegarty K, Blashki G. A systematic review of complex system interventions designed to increase recovery from depression in primary care. BMC Health Serv Res 2006;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525. [DOI] [PubMed] [Google Scholar]
- 12.Strong V, Waters R, Hibberd C, Murray G, Wall L, Walker J, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet 2008;372:40-48. [DOI] [PubMed] [Google Scholar]
- 13.NICE. Depression in adults with a chronic physical health problem. The NICE Guideline on Treatment and Management. National Clinical Practice Guideline 91. British Psychological Society and Royal College of Psychiatrists, 2010. [PubMed]
- 14.Coventry PA, Lovell K, Dickens C, Bower P, Chew-Graham C, Cherrington A, et al. Collaborative Interventions for Circulation and Depression (COINCIDE): study protocol for a cluster randomized controlled trial of collaborative care for depression in people with diabetes and/or coronary heart disease. Trials 2012;13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coventry PA, Lovell K, Dickens C, Bower P, Chew-Graham C, Cherrington A, et al. Update on the collaborative interventions for circulation and depression (COINCIDE) trial: changes to planned methodology of a cluster randomized controlled trial of collaborative care for depression in people with diabetes and/or coronary heart disease. Trials 2013;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ 2005;330:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department for Communities and Local Government. English indices of deprivation 2010. Stationery Office, 2011.
- 19.Brown S, Thorpe H, Hawkins K, Brown J. Minimization—reducing predictability for multi-centre trials whilst retaining balance within centre. Stat Med 2005;24:3715-27. [DOI] [PubMed] [Google Scholar]
- 20.NICE. Depression in adults with a chronic physical health problem. Treatment and management. NICE, 2009. [DOI] [PubMed]
- 21.Myles P, Rushforth D. The complete guide to primary care mental health. Constable and Robinson, 2007.
- 22.Briddon J, Baguley C, Webber M. The ABC-E Model of Emotion: a bio-psychosocial model for primary mental health care. J Mental Health Train Educ Pract 2008;3:12-21. [Google Scholar]
- 23.NICE. Depression. The treatment and management of depression in adults (updated edition). NICE Clinical Guidelines, No 90. British Psychological Society, 2010. [PubMed]
- 24.Knowles S, Chew-Graham C, Coupe N, Adeyemi I, Keyworth C, Thampy H, et al. Better together? a naturalistic qualitative study of inter-professional working in collaborative care for co-morbid depression and physical health problems. Implement Sci 2013;8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turpin G, Wheeler S. IAPT supervision guidelines. IAPT, 2011.
- 26.Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the scl-90: a study in construct validation. J Clin Psychol 1977;33:981-89. [Google Scholar]
- 27.Spitzer RL, Kroenke K, Williams JW, Löwe B. A brief measure for assessing generalized anxiety disorder: the gad-7. Arch Intern Med 2006;166:1092-97. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CR, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. J Cardiopulm Rehab 2003;23:398-403. [DOI] [PubMed] [Google Scholar]
- 29.Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res 2004;13:299-310. [DOI] [PubMed] [Google Scholar]
- 30.DCCT Research Group. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). The DCCT Research Group. Diabetes Care 1988;11:725-32. [DOI] [PubMed] [Google Scholar]
- 31.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333-41. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol 1996;11(suppl 3):89-95. [DOI] [PubMed] [Google Scholar]
- 33.Osborne RH, Elsworth GR, Whitfield K. The health education impact questionnaire (heiQ): an outcomes and evaluation measure for patient education and self-management interventions for people with chronic conditions. Patient Educ Couns 2007;66:192-201. [DOI] [PubMed] [Google Scholar]
- 34.Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Sage, 1996.
- 35.Gibbons CJ, Kenning C, Coventry P, Bee P, Fisher L, Bower P. Development of a multimorbidity illness perceptions scale (MULTIPleS). PLoS One 2013. 8:e81852. [DOI] [PMC free article] [PubMed]
- 36.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the patient assessment of chronic illness care (PACIC). Med Care 2005;43:436-44. [DOI] [PubMed] [Google Scholar]
- 37.Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann 1982;5:233-7. [DOI] [PubMed] [Google Scholar]
- 38.Rick J, Rowe K, Hann M, Sibbald B, Reeves D, Roland M, et al. Psychometric properties of the patient assessment of chronic illness care measure: acceptability, reliability and validity in United Kingdom patients with long-term conditions. BMC Health Serv Res 2012;12:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lovell K, Bower P, Richards D, Barkham M, Sibbald B, Roberts C, et al. Developing guided self-help for depression using the Medical Research Council complex interventions framework: a description of the modelling phase and results of an exploratory randomised controlled trial. BMC Psychiatry 2008;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
- 41.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics 2000;56:645-6. [DOI] [PubMed] [Google Scholar]
- 42.Bayliss EA, Ellis JL, Steiner JF. Seniors’ self-reported multimorbidity captured biopsychosocial factors not incorporated into two other data-based morbidity measures. J Clin Epidemiol 2009;62:550-7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. [DOI] [PubMed] [Google Scholar]
- 44.Brockmeier LL, Kromrey JD, Hogarty KY. Nonrandomly missing data in multiple regression analysis: an empirical comparison of ten missing data treatments. Multiple Linear Regression Viewpoints 2003;29:8-29. [Google Scholar]
- 45.Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347;f4913. [DOI] [PMC free article] [PubMed]
- 46.Mercer SW, Gunn J, Bower P, Wyke S, Guthrie B. Managing patients with mental and physical multimorbidity. BMJ 2012;345;e5559. [DOI] [PubMed]
- 47.Mercer SW, Guthrie B, Furler J, Watt GC, Hart JT. Multimorbidity and the inverse care law in primary care. BMJ 2012;344:e4152. [DOI] [PubMed] [Google Scholar]
- 48.Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet 2012;380:7-9. [DOI] [PubMed] [Google Scholar]
- 49.Von Korff M, Katon WJ, Lin EH, Ciechanowski P, Peterson D, Ludman EJ, et al. Functional outcomes of multi-condition collaborative care and successful ageing: results of randomised trial. BMJ 2011;343:d6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin EH, VonKorff M, Russo J, Katon W, Simon GE, Unutzer J, et al. Can depression treatment in primary care reduce disability? A stepped care approach. Arch Fam Med 2000;9:1052-8. [DOI] [PubMed] [Google Scholar]
- 51.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106-16. [DOI] [PubMed] [Google Scholar]
- 52.Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coventry PA, Hudson JL, Kontopantelis E, Archer J, Richards DA, Gilbody S, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One 2014;9:e108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chalder M, Wiles NJ, Campbell J, Hollinghurst SP, Haase AM, Taylor AH, et al. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. BMJ 2012;344:e2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katon WJ, Lin EHB, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilbody S, House AO, Sheldon TA. Screening and case finding instruments for depression. Cochrane Database Syst Rev 2005;4:CD002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042-9. [DOI] [PubMed] [Google Scholar]
- 58.Morgan MA, Coates MJ, Dunbar JA, Reddy P, Schlicht K, Fuller J. The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial. BMJ Open 2013;3:e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ 2012;344:e1553. [DOI] [PubMed] [Google Scholar]
- 60.Cape J, Whittington C, Buszewicz M, Wallace P, Underwood L. Brief psychological therapies for anxiety and depression in primary care: meta-analysis and meta-regression. BMC Med 2010;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Unutzer J, Katon W, Callahan CM, Williams JW Jr, Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836-45. [DOI] [PubMed] [Google Scholar]
- 62.NIHR Collaboration for Leadership in Applied Health Research and Care Greater Manchester.http://clahrc-gm.nihr.ac.uk/our-work/patient-centred-care/mental-health-coincide/.
- 63.Lancet Global Health Group. Scale up services for mental disorders: a call for action. Lancet 2007;370:1241-52. [DOI] [PubMed] [Google Scholar]
- 64.Sharpe M, Walker J, Holm Hansen C, Martin P, Symeonides S, Gourley C, et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): a multicentre randomised controlled effectiveness trial. Lancet 2014;384:1099-108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: COINCIDE Patient Manual
Appendix 2: COINCIDE Workbook
Appendix 3: COINCIDE PWP Manual