Figure 1.
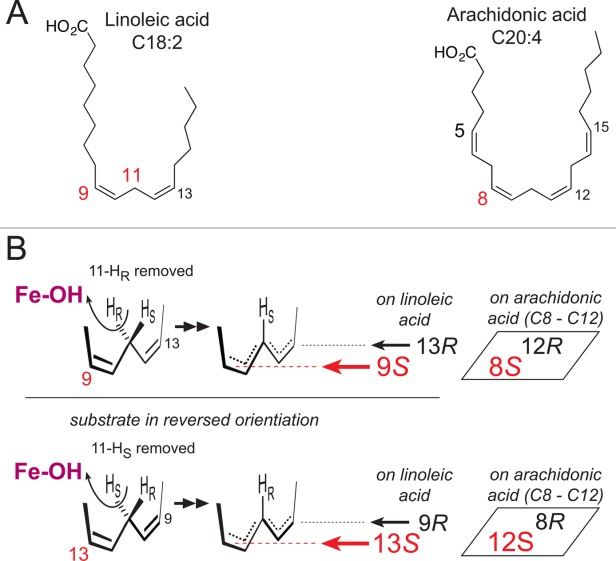
Figure The basis of LOX regio- and stereospecificity. (A)The structures of linoleic acid (18:2) and arachidonic acid (20:4). (B) A perspective view on LOX catalysis showing the reacting pentadiene. Top left: The catalytic iron (Fe-OH) abstracts the pro-R hydrogen from C11 and O2 is added antarafacially in the 9S position (in red, in the foreground).15 Lower left: with linoleic acid in a reversed head-to-tail orientation, the identical stereochemical relationships involve pro-S hydrogen abstraction at C11 and oxygenation in the 13S position (in red, in the foreground).15 Formation of the corresponding 9R or 13R configuration products entails antarafacial reaction of O2 at the opposite end of the pentadiene.16 In boxes on the right side: an example of the corresponding positional specificities of arachidonic acid, illustrating the positions around the 8–12 pentadiene on arachidonic acid.
