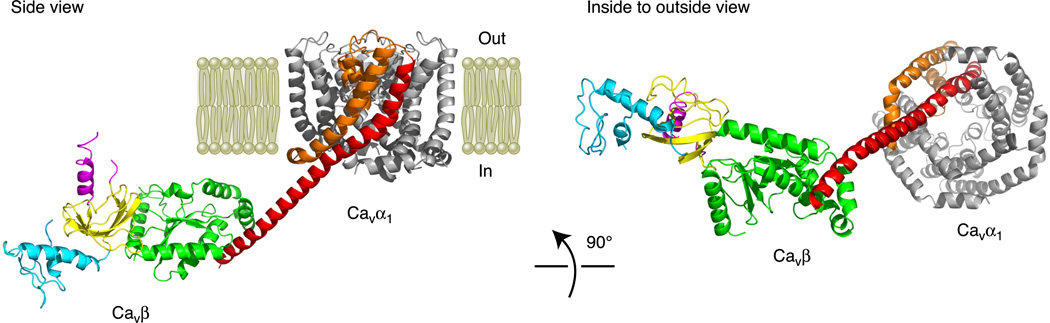
Fig. 4.
Structural model of a partial Cavα1/Cavβ complex on the plasma membrane. A side view and an inside-to-outside view are presented. The partial structure of Cavα1 includes only the S5, P-loop, and S6 segments and is based on a Cavα1 homology model developed in Stary et al. (411). IS5 is colored orange, and IS6 is red. The IS6-AID linker from Cav1.2 is modeled as an α-helix and is joined with IS6 at its NH2 terminus and the AID at its COOH terminus. The structure of Cavβ is based on the crystal structure of the β4 core region (84) and the NMR structure of the β4 NH2 terminus (451); there is no Cavβ COOH terminus. Since the structure of the β4-AID complex is not available, we docked the AID to β4 based on the crystal structure of the β3 core-AID complex (84). The regions of Cavβ are color coded as in Figures 2 and 3 (NH2 terminus in light blue, SH3 in gold, HOOK in purple, and GK in green).