Figure 7. Hypothalamic Syt4-OXT links HFD feeding to disease.
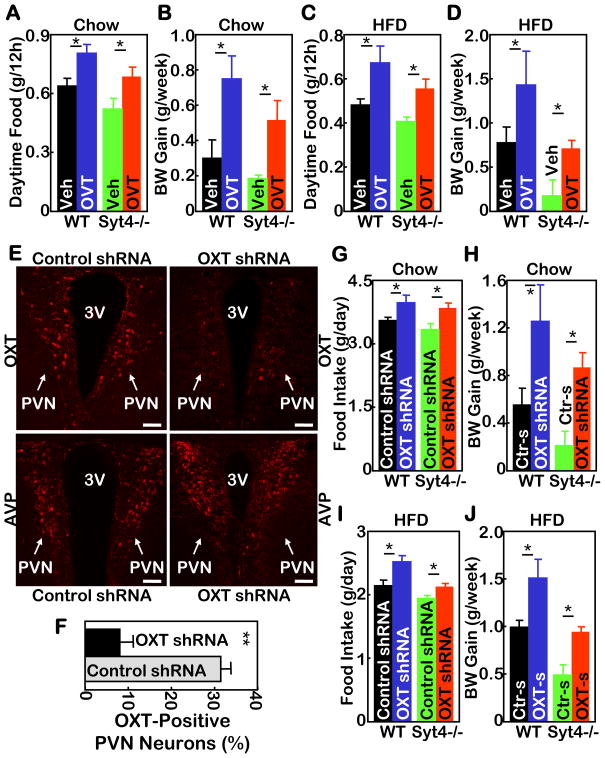
A–D. Syt4−/− mice and WT littermate controls were maintained on a normal chow (A&B) vs. a HFD (C&D) for 3 months since weaning, and subsequently implanted with cannula into the third ventricle. After 2 weeks of post-operative recovery, mice received daily third ventricle injections of OVT (4 μg) vs. vehicle (Veh) for 2 weeks, and were monitored for daily 12-hour daytime food intake post injection (A&C) and weekly body weight (BW) gain (B&D) during the 2-week treatment period. *P<0.05, n= 5–6 per group. Mice in all these experiments were males.
E–J. Syt4−/− mice and WT littermate controls were maintained on a normal chow (E–H) vs. a HFD (I&J) for 5 months since weaning, and subsequently received bilateral intra-PVN injection of OXT shRNA (OXT-s) lentiviruses or control shRNA (Ctr-s) lentiviruses. E&F: Lentivirus-mediated OXT ablation was assessed by OXT immunostaining (E upper panels) and quantitated by counting OXT-expressing neurons in the serial PVN sections (F). AVP immunostaining of matched PVN sections (E lower panels) was used as a control. 3V: third ventricle; scale bar=50 μm. G–J: Mice were monitored for daily food intake (G&I) and weekly BW gain (H&J) during a 3-week follow-up. *P<0.05, n= 4–7 per group. Mice in all these experiments were males.