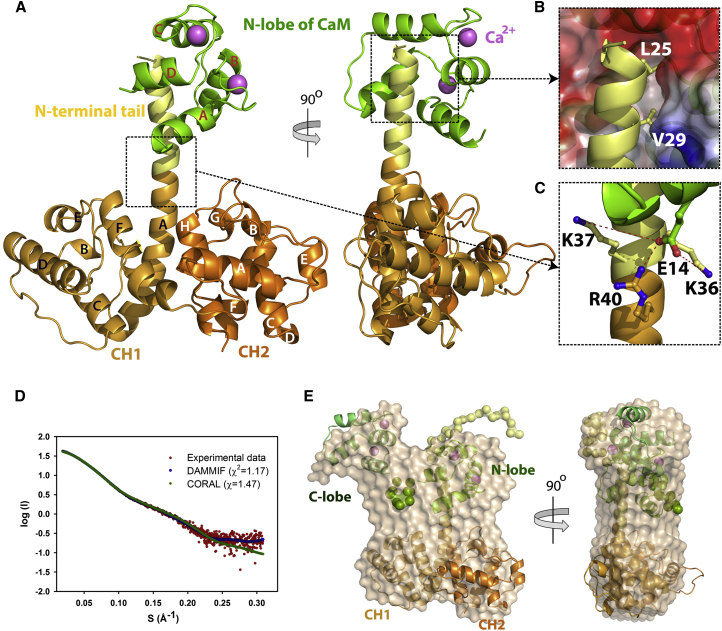
Figure 4.
Structure of the P1aABD/CaM Complex
(A) Crystal structure of the P1aABDΔ22/CaMNL complex. The complex is displayed in two orientations (90° rotated along the y axis). CH1 and CH2 in the ABD are colored respectively in bronze and orange, the N-ter tail of P1a in yellow and CaMNL in green. Ca2+ is depicted as a violet sphere.
(B) The binding interface of the P1aABDΔ22/CaMNL complex. CaMNL is shown according to electrostatic surface potential, with blue and red depicting positive and negative electrostatic potentials, respectively. Two residues of P1a (L25 and V29) important for interaction with CaM are buried in the hydrophobic cleft of CaMNL.
(C) A salt bridge between E14 of CaM and R40 of plectin (2.7 Å apart). XL-MS analysis shows that CaM E14 is crosslinked with K36 (5.7 Å apart) and K37 of plectin (7.0 Å).
(D) Experimental SAXS data of the P1aABD/CaM complex is shown in red; calculated scattering curves from SAXS models are individually presented in green (rigid-body modeling by CORAL) and blue (ab initio modeling by DAMMIF) lines.
(E) The ab initio molecular shape of the P1aABD/CaM complex (shown in transparent beads) was superimposed over the SAXS-derived rigid-body model of the complex. Flexible residues (1–21 residues of P1aABD and 74–82 residues of CaM) were modeled as dummy residues (colored spheres).