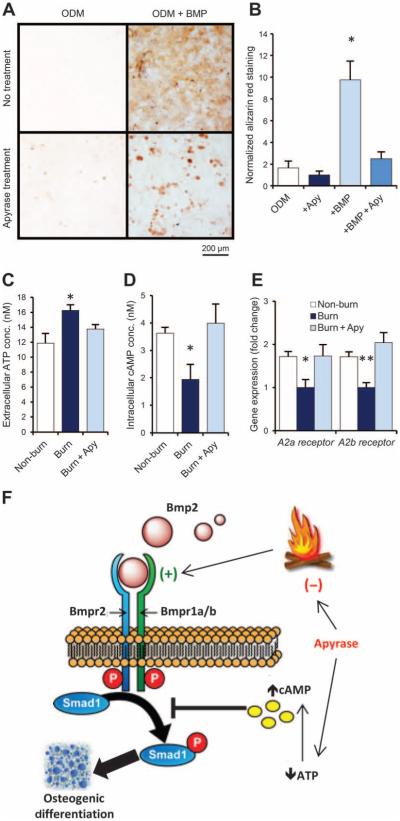
Fig. 4. Apyrase mitigates burn injury–induced osteogenic differentiation of MSCs by modulating ATP signaling.
(A and B) Alizarin red stain (A) and quantification of osteoid deposition (B) of mMSCs collected from adipose tissue of untreated C57BL/6 mice and exposed to ODM with or without supplementation with recombinant BMP-2 ligand (200 ng/ml) and/or apyrase (4 U/ml). Scale bar, 200 μm. Data are means ± SD (n = 3 per group). P = 0.030 (ANOVA). (C) ATP assay for extracellular ATP concentrations in mMSC cultures harvested from the adipose tissue of mice 2 hours after burn injury, burn injury + topical apyrase treatment, or non-burn control (exposure to room temperature water). Data are means ± SD (n = 3 per group). P = 0.038 (ANOVA). (D) cAMP assay for intracellular cAMP from the same cells, showing that intracellular cAMP is reduced in MSCs from burn mice and up-regulated with apyrase treatment. Data are means ± SD (n = 3 per group). P = 0.018 (ANOVA). (E) Gene expression for the adenosine receptors A2a and A2b in the same mMSCs, assessed by qRT-PCR of mRNA collected after 7 days of exposure to ODM. Both adenosine receptors were down-regulated after burn injury, but this effect was absent in mice that received apyrase treatment or no burn. Data are means ± SD (n = 3 per group). A2a receptor, P = 0.011; A2b receptor, P = 0.004 (ANOVA). (F) Schematic showing BMP and ATP signaling interactions in HO formation after burn injury. Apyrase application after burn injury limits ATP and BMP signaling, thereby reducing the otherwise up-regulated osteogenicity of MSCs. *P < 0.05, **P < 0.01.