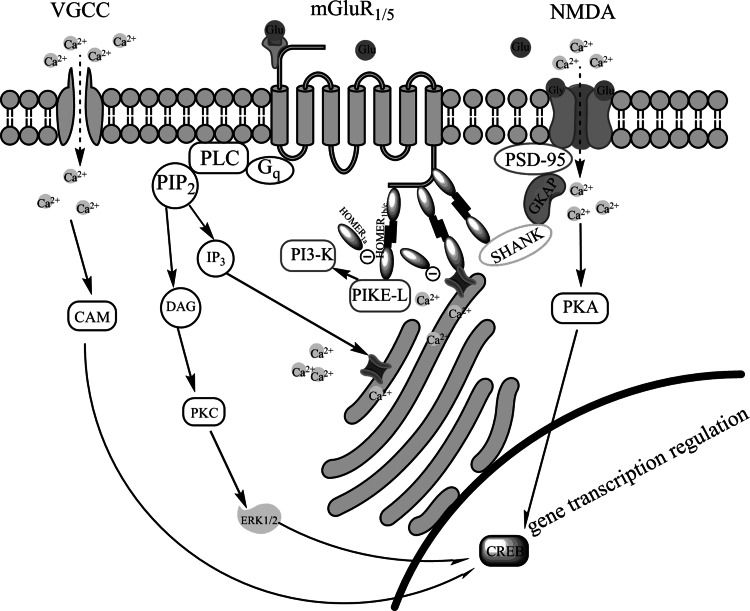
Fig. 1.
Interactions between mGluR1/5 and NMDA receptors and their signaling pathways in the postsynaptic neuron. NMDA receptors are linked to mGluR1/5 receptors through complex composed of cytoplasmatic proteins PSD-95, guanylate kinase-associated protein (GKAP), Shank protein and Homer1b/c. Stimulation of mGluR1/5 receptors activates phospholipase C (PLC) and thereby leads to enhanced production of inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) forms phosphatidylinositol-4,5-biphosphate (PIP2) and potentiates L-type voltage-dependent calcium channels (VGCC). Elevated IP3 level mobilizes Ca2+ release from internal stores. DAG contributes to activation protein kinase C (PKC) and subsequently extracellular signal-regulated protein kinases ERK1/2 phosphorylation. mGluR1/5 receptors are also coupled by means of Homer1b/c and phosphoinositide-3-kinase enhancer (PIKE-L) to phosphoinositide-3-kinase (PI3-K). All interactions involving long isoforms of Homer are disrupted by the short isoform Homer1a and thereby affect mGluR1/5 signaling. Summarizing activation of mGluR5 and NMDA receptors leads to phosphorylation transcription factors, therein cAMP response element-binding protein (CREB) and changes in gene expression