FIGURE 1.
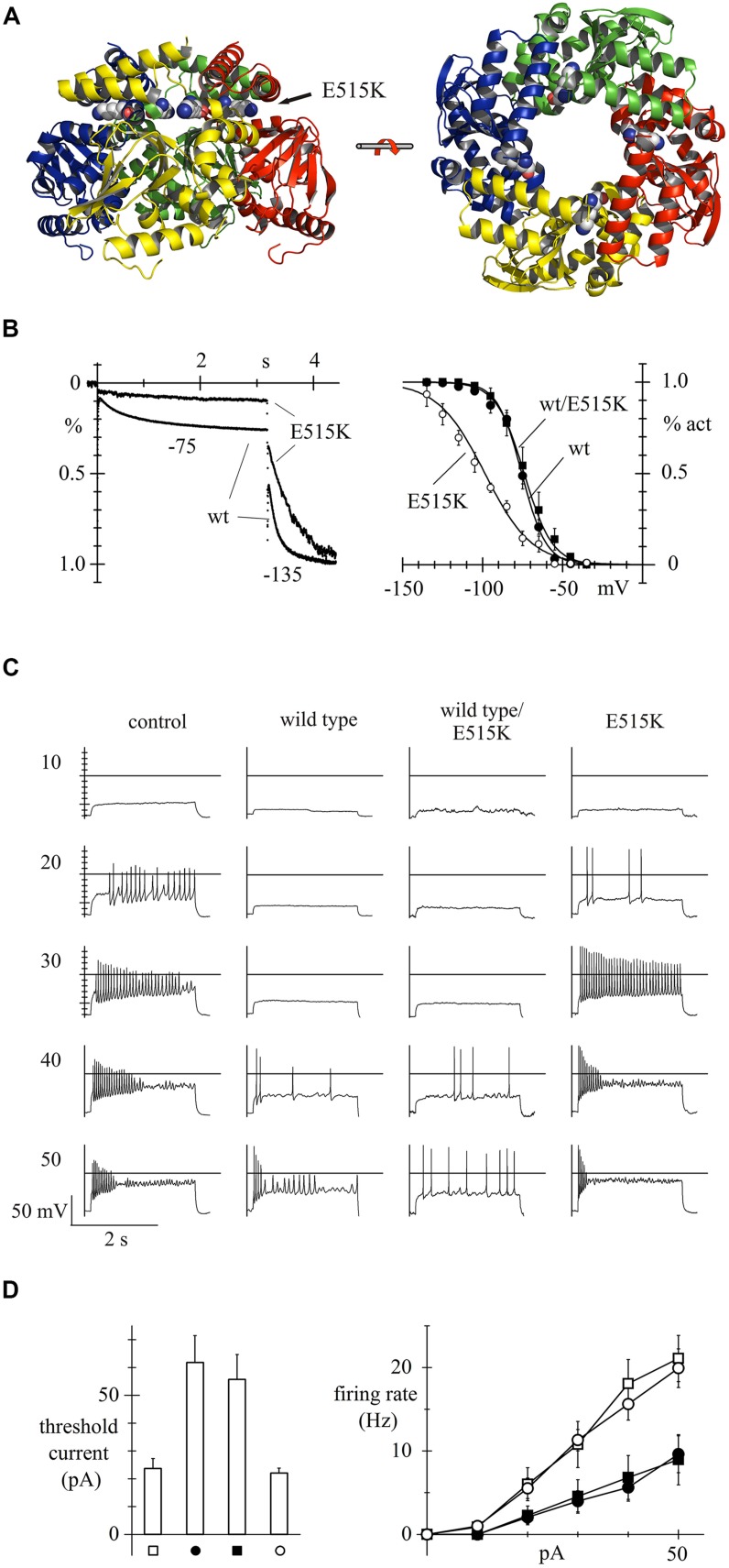
Human HCN2 E515K mutation and its effect on neuronal excitability. 3D structure of hHCN2 C-linker and cyclic nucleotide binding domain (CNBD) tetrameric domains based on X-ray crystallographic data (Zagotta et al., 2003) and plotted as ribbon representation. Views shown are perpendicular (left) and parallel (right) to fourfold axis; E515 residues are drawn as space filling plots; the E515K mutation is located in the C-linker region (A). When overexpressed in neonatal rat cortical neurons, the E515K homozygous mutation leads to a large decrease of Ih in the voltage range of current activation (left), due to a large negative shift of the activation curve (right), when compared to both the wild-type (wt) and the heterozygous mutation channel (wt/E515K) (B). Measurement of the firing rate due to injection of various degrees of depolarizing currents (range 10–50 pA) shows that neonatal rat cortical neurons transfected with either wild type or heterozygous wild-type/mutant channels respond with a much-reduced excitability than neurons transfected with empty vectors (controls) or with homozygous E515K mutant channels (C). Mean threshold current required to trigger action potential firing and mean rate of firing recorded upon current injection in control, wt, wt/E515K, and E515K cells. Neuronal models transfected with homozygous E515K mutant channels, like empty vectors, show a significantly lower threshold current to trigger neuronal action potential and a higher rate of firing action potential, upon depolarizing current injection (D). Data from (DiFrancesco et al., 2011), with permission.