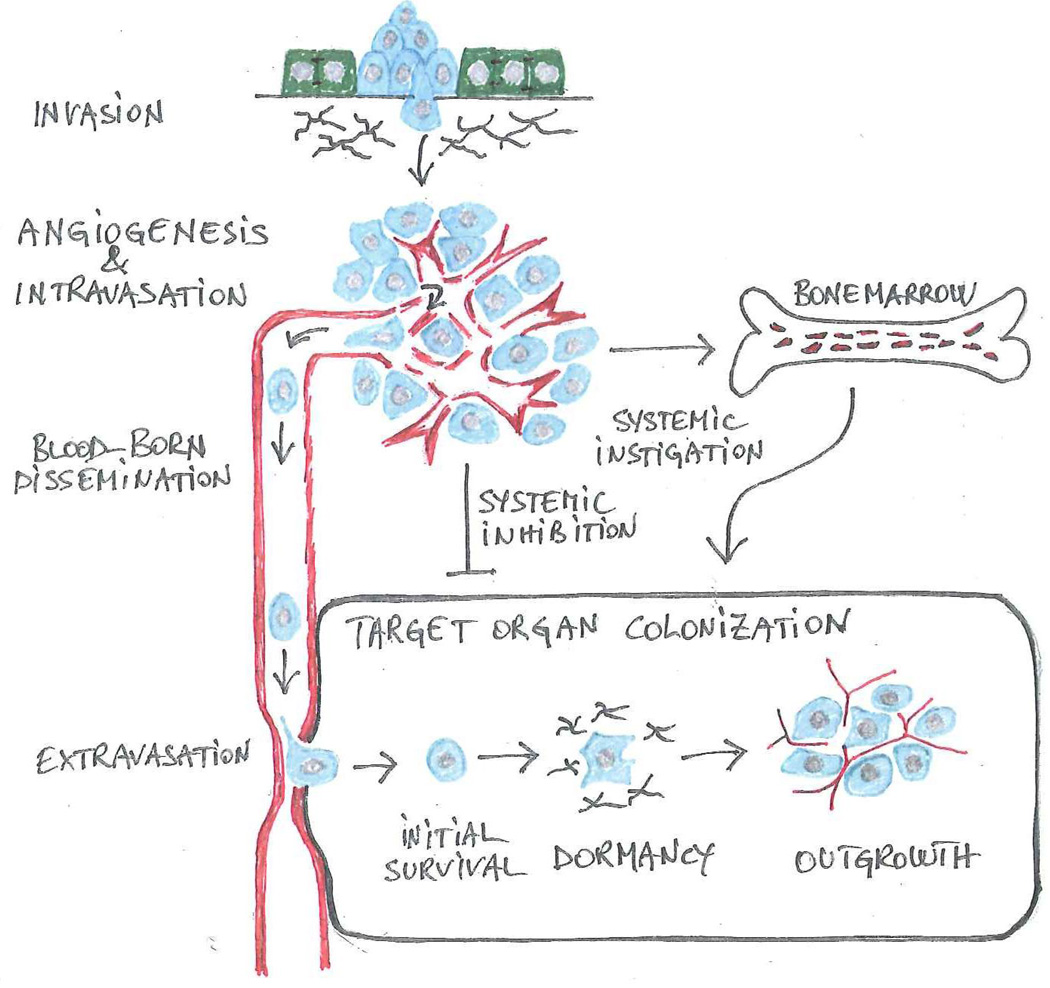
Figure 1. The invasion-metastasis cascade.
Genetic and epigenetic alterations endow cancer cells with the capabilities that enable them to negotiate the sequential steps comprising the invasion-metastasis cascade. A partial or complete EMT allows individual carcinoma cells or small groups of carcinoma cells to disassociate from adjacent epithelial cells and to invade into the underlying interstitial matrix (invasion). In a tightly linked process, cancer cells coopt a wide spectrum of host cells to create a permissive microenvironment. Upon recruiting angiogenic endothelial cells and inducing the development of a defective vasculature (angiogenesis), cancer cells enter into the circulation (intravasation) and disseminate via the bloodstream (blood-born dissemination). In a variation from the predominant sequence depicted here, cancer cells enter into lymphatic vessels and colonize loco-regional lymphnodes prior to entering into the blood stream. Upon arresting in the microcirculation, cancer cells disrupt the endothelial junctions and penetrate into the stroma of the target organ (extravasation). In the final step, colonization, they resist apoptosis (initial survival), undergo a variable period of dormancy (dormancy) and finally outgrow into macroscopic lesions (outgrowth). In order to colonize a target organ, cancer cells need to mold a permissive microenvironment. In certain cases, systemic signals retard the vascularization of micrometastases (systemic inhibition), potentially explaining why surgical resection of the primary tumor may induce rapid outgrowth of metastatic lesions (Demicheli et al., 2007). Other systemic signals are proposed to spur metastatic outgrowth via the recruitment of bone marrow-derived hematopoietic cells (systemic instigation).