Abstract
Background
Decreased expression of phospholipase C-β1 (PLC-β1) has been observed in the brains of patients with schizophrenia, but, to our knowledge, no studies have shown a possible association between this altered PLC-β1 expression and the pathogenesis of schizophrenia. Although PLC-β1-null (PLC-β1−/−) mice exhibit multiple endophenotypes of schizophrenia, it remains unclear how regional decreases in PLC-β1 expression in the brain contribute to specific behavioural defects.
Methods
We selectively knocked down PLC-β1 in the medial prefrontal cortex (mPFC) using a small hairpin RNA strategy in mice.
Results
Silencing PLC-β1 in the mPFC resulted in working memory deficits, as assayed using the delayed non-match-to-sample T-maze task. Notably, however, other schizophrenia- related behaviours observed in PLC-β1−/− mice, including phenotypes related to locomotor activity, sociability and sensorimotor gating, were normal in PLC-β1 knockdown mice.
Limitations
Phenotypes of PLC-β1 knockdown mice, such as locomotion, anxiety and sensorimotor gating, have already been published in our previous studies. Further, the neural mechanisms underlying the working memory deficit in mice may be different from those in human schizophrenia.
Conclusion
These results indicate that PLC-β1 signalling in the mPFC is required for working memory. Importantly, these results support the notion that the decrease in PLC-β1 expression in the brains of patients with schizophrenia is a pathogenically relevant molecular marker of the disorder.
Introduction
Understanding the pathogenic mechanism of a mental disorder in terms of molecular changes in specific neural circuitries presents one of the major challenges in neurobiological studies of psychiatric diseases. Phospholipase C-β1 (PLC-β1) is a phosphoinositide-specific phosphodiesterase that is readily activated by Gq protein-coupled neurotransmitter receptors, such as muscarinic acetylcholine receptors, group 1 metabotropic glutamate receptors and serotonergic receptors.1–4 PLC-β1 hydrolyzes phosphatidylinositol 4,5-bisphosphate to produce a pair of second messengers: diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3).5 In general, DAG stimulates the phosphorylating enzyme, protein kinase C, whereas IP3 mobilizes Ca2+ from intracellular endoplasmic reticulum stores to the cytoplasm.6 PLC-β1 expression is abundant in the cerebral cortex, although it is widely distributed in many brain areas.3,7,8 Several studies have previously reported that PLC-β1-related pathways are involved in various neurophysiological functions.4,9–12 Moreover, decreased expression of PLC-β1 has been detected in the brains of patients with schizophrenia.13–18
Schizophrenia symptoms are categorized into 3 major types19,20 — positive, negative and cognitive — and a majority of these symptoms have been observed as endophenotypes in PLC-β1-null (PLC-β1−/−) mice.9–12 These symptoms include increased locomotion, impaired social behaviour, impaired prepulse inhibition (PPI), and impaired working memory.9–12 Furthermore, reports of altered PLC-β1 expression in the brains of patients with schizophrenia, such as in the dorsolateral prefrontal cortex (DLPFC), have suggested the possible pathogenic involvement of PLC-β1 in schizophrenia.13,14,21 Nonetheless, a causal link between this biochemical change and the manifestation of diverse schizophrenic symptoms has not been demonstrated.
Working memory processes and provides transitory storage of information and thus plays a critical role in cognition. Without its proper operation, cognitive life would not be possible.22 Working memory deficits have been previously reported in a number of neurologic and psychiatric disorders, including schizophrenia, Alzheimer disease, Parkinson disease and major depression.23–25 Neuroimaging studies in humans have revealed that working memory processing is widely distributed throughout multiple brain regions, including the DLPFC. Signals associated with working memory are reduced in the DLPFC of patients with schizophrenia.26,27 In addition, DLPFC damage is associated with a deficit of working memory in human patients with brain damage.28 However, molecular and cellular mechanisms responsible for working memory in the DLPFC of the human brain are poorly understood.
The human DLPFC has been shown to have anatomic as well as functional homology to the mouse mPFC, which consists of prelimbic and infralimbic cortices.5,29–31 Therefore, to define a specific role for PLC-β1 in the medial prefrontal cortex (mPFC) in the pathogenesis of schizophrenia, we produced mPFC-selective PLC-β1-knockdown mice and carried out a battery of behavioural tests designed to examine behaviours relevant to the schizophrenic endophenotypes of PLC-β1−/− mice.
Methods
Animals
Adult male PLC-β1−/− and wild-type littermate mice (12–16 wk of age) in a B6 × 129 F1 background were obtained by mating parental strain C57BL/6J (N26) PLC-β1+/− and 129S4/SvJae (N39) PLC-β1+/− mice.3 Mice were maintained with free access to food and water under a 12-hour light/dark cycle. Animal care and experimental procedures followed the guidelines of the Institutional Animal Care and Use Committee of the Korea Institute of Science and Technology.
Verification of shRNA-mediated knockdown of PLC-β1
Lentiviral vectors were created as described previously.32 Briefly, a lentiviral vector expressing a small hairpin RNA (shRNA) targeting PLC-β1 mRNA was constructed by inserting synthetic double-stranded oligonucleotides into a shSynLenti4.4G lentiviral vector backbone containing a U6 small nuclear RNA promoter and a GFP-IRES-puror gene cassette under the control of the synapsin-1 promoter. Eleven different shSynLenti4.4G lentiviral vectors encoding PLC-β1-specific shRNA (shPLC-β1) expression cassettes were tested. Validation of shRNA-expressing lentiviral vectors was performed as described previously.32 Of the 11 tested shRNAs, shPLC-β1–11 (target sequence, 5′-CCTCCAGTGAGGAGA-TAGAAA-3′) effectively reduced PLC-β1 expression levels in C2C12 cells transfected with shRNA-expressing lentiviral vectors and was chosen for subsequent in vivo experiments. The scrambled shRNA (shSCR) sequence, 5′-AATCG-CATAGCGTATGCCGTT-3′, was used to construct a nontargeting control virus.
Injection of shPLC-β1-expressing lentivirus into the mPFC
After anesthetizing wild-type mice with 2% Avertin (tribromoethyl alcohol/tertiary amyl alcohol; Aldrich), 0.3 μL of a high-titer lentiviral preparation (109 TU/μL) was bilaterally injected into the mPFC (+1.6 anterior–posterior, ± 0.3 mediolateral, –2.6 dorsoventral) at a rate of 0.05 μL/min using a Hamilton syringe connected to a microinjection pump (sp100i; World Precision Instruments).
Working memory
The delayed non-match-to-sample (DNMTS) T-maze test was carried out as reported previously with minor modifications.33,34 Mice were tested using 6 trials per day, each consisting of a forced and a choice run. In the forced run, the mouse was placed in the open arm containing a food pellet (20 mg dustless sugar pellets; Bioserv) at one end. As soon as the mouse ate the food pellet, it was placed in a dark transfer cage for a 4-second delay. In the choice run, the mouse was allowed to choose between 2 open arms. A correct choice was scored when the mouse visited the alternate arm in the choice run. The criterion for successful completion of the task was 11 correct choices out of 12 consecutive trials (92%). Each mouse was tested for up to 15 days, during which the sequence of baiting sides (right or left) was randomized. Right/left T-maze discrimination was tested as described previously34 as a positive control for any deficit in the DNMTS T-maze task. The right/left discrimination version of the T-maze was carried out with the same apparatus using the same conditions as those used for the habituation procedure, but the rule for acquiring the task was different. In this case, the baiting target was fixed at one side — left or right — for all trials throughout the entire experimental period. The data were statistically analyzed using a 2-tailed Student t test, a Pearson correlation test and a 2-sample Kolmogorov–Smirnov test.
Locomotor activity and anxiety
Three behavioural assays for locomotor activity and anxiety were performed as described previously.32 Mice were placed in the central square region (20 × 20 cm) of an open field box (40 × 40 × 40 cm), and the extent of spontaneous movement over the course of 1 hour was analyzed using the Ethovision 3.1 program (Noldus Information Technology). Mice were placed in an elevated (30 cm above floor level) plus-maze with 2 opposite open arms (45 × 5 cm each) and 2 opposite closed arms (45 × 5 cm each) with walls 15 cm high. We measured the numbers of entries into individual arms and the time spent on individual arms for 5 minutes. The apparatus used for the light/dark transition test consisted of a cage (25 × 40 × 20 cm) divided into 2 compartments by a black partition containing a small opening that allowed the mice to move between compartments. One of the boxes was darkened; the other was brightly lit. Mice were placed in the dark compartment and allowed to move freely. We recorded the number of transitions between the 2 compartments and the time spent in each chamber for 5 minutes. Statistical significance was analyzed by 1-way repeated-measures analysis of variance (ANOVA) followed by a Tukey post hoc test and 2-tailed Student t test for paired comparisons.
Acoustic startle response and prepulse inhibition of acoustic startle
Acoustic startle response (ASR) and prepulse inhibition (PPI) tests were administered as reported previously.35 Briefly, mice were placed into a cylinder and acclimated to the startle chamber (San Diego Instruments) for 10 minutes. The test involved a series of 7 blocks of 8 trials (56 trials in total). The 8 trial types were a no stimulus trial; a startle stimulus alone trial in which a 40 ms, 120-dB burst of white noise was presented; 3 different prepulse–pulse (PP) trials in which a 20 ms prepulse stimulus of different intensities (74-, 82- and 90-dB white noise) were presented; and 3 different trials of a PP plus a startle stimulus in which a 20 ms prepulse stimulus of different intensities (74-, 82- and 90-dB white noise) preceded the onset of the 120-dB startle stimulus by 100 ms. We calculated percent PPI as described previously.35 We assessed statistical significance using repeated-measures ANOVA and a 2-tailed Student t test.
Social behaviour test
The social behaviour test was administered using previously described methods with modifications.36,37 The apparatus used for the social behaviour task was an open-field box (a square, white acrylic box, 40 × 40 × 40 cm) containing 2 wire cages (JEUNDO Bio & Plant) placed at opposite corners. The wire cages were 11 cm high, with a bottom diameter of 10.5 cm and bars spaced 1 cm apart. This apparatus allowed mice to make nose contact between the bars, but prevented further interaction. In the habituation period, a test mouse was placed in the social behaviour apparatus and allowed to explore for 1 hour under dim light conditions. A wild-type mouse that had no prior contact with the test mouse was enclosed in 1 of the wire cages. Then, novel objects (3 Falcon tube lids) were placed in the other wire cage. The test mouse was allowed to explore the social behaviour apparatus for 10 minutes. The sniffing time for each wire cage was the duration of time that the nose of the test mouse was in contact with each wire cage, measured using a stopwatch. Total sniffing time was obtained by summing the sniffing time for each wire cage. We used a 2-tailed Student t test and 1-way ANOVA to assess statistical significance.
Histology for PLC-β1 expression in the mPFC
To verify PLC-β1 expression in the mPFC, we performed histology as described previously.38 Every sixth section in the series throughout the entire mPFC from selected mice was used for immunohistochemistry. Sections were stained by incubation with rabbit anti-PLC-β1 primary antibody (1:100; Santa Cruz Biotechnology) and then with horseradish peroxidase (HRP)-conjugated secondary antibody. Immunoreactivity was visualized with the HRP substrate 3, 3′-diaminobenzidine (DAB) in 0.1 M Tris buffer. Changes in PLC-β1-positive neuron morphology and expression of PLC-β1 induced by shPLC-β1 in the mPFC were also evaluated by immunofluorescence staining using rabbit anti-PLC-β1 primary antibody (1:100) and Cy3-conjugated secondary antisera (1:200). Sections were mounted in Vectashield mounting media with or without diamidino-2-phenylindole (DAPI; Vector Laboratories). Thereafter, images were captured and analyzed using Olympus DP72 digital camera and DP2-BSW microscopic digital camera software. Figures were prepared using Adobe Photoshop 7.0. We quantified PLC-β1 immunofluorescence in RNA interference-mediated PLC-β1 knockdown mice as described previously.32 We used 1-way ANOVA to assess statistical significance.
Results
Endogenous expression of PLC-β1 in the mPFC
A previous study using in situ hybridization techniques reported abundant expression of PLC-β1 mRNA in the mPFC of mice.7 We performed immunohistochemical staining to determine the level of PLC-β1 protein in the mPFC of mouse brains. PLC-β1 was highly expressed in the mPFC of wild-type mice (see the Appendix, Fig. S1A, available at jpn.ca). As expected, no expression of PLC-β1 protein was detected in PLC-β1−/− mice (Appendix, Fig. S1B and D). At higher magnification, the morphology of PLC-β1-expressing cells in the mPFC was consistent with that of neuronal cells (Appendix, Fig. S1C). PLC-β1 expression was shown mainly in the cytoplasm within the neuronal cell body by immunofluorescence staining of PLC-β1 with DAPI (Fig. 1C).
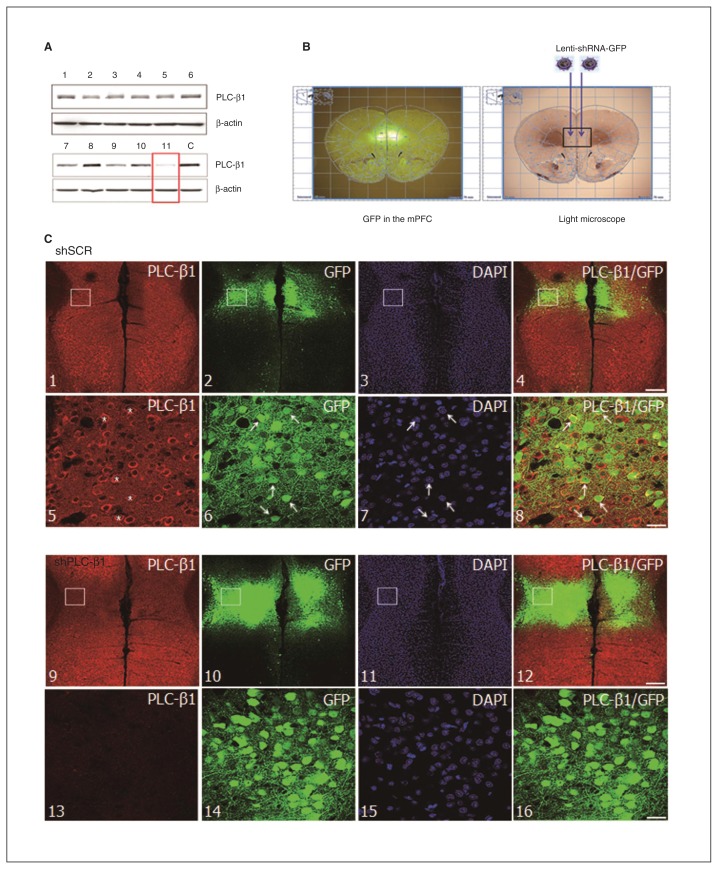
Fig. 1.
Phospholipase C-β1 (PLC-β1) knockdown in the medial prefrontal cortex (mPFC) by injection of small hairpin RNA (shRNA) lenti-virus. (A) PLC-β1-specific shRNA vectors reduced endogenous PLC-β1 expression in C2C12 cell cultures. An antibody against β-actin was used as an internal control for each sample. Lane C is a negative control. (B) Lentiviral vectors expressing shRNA for PLC-β1 (shPLC-β1) or a scrambled sequence (shSCR) were bilaterally injected into the mPFC of a mouse brain (right panel). Green fluorescent protein (GFP) expression under the control of the synapsin-1 promoter was detected in the mPFC (left panel). (C) Immunofluorescence staining for PLC-β1 expression in the mPFC of mice injected with shSCR (panels 1–8) and shPLC-β1 (panels 9–16). Panels 5–8 and 13–16 are higher magnification images corresponding to the rectangles in panels 1–4 and 9–12, respectively. *PLCβ-1-positive neurons in mice injected with shSCR. Scale bars: 100 μm (panels 1–4, 9–12) and 18.8 μm (panels 5–8, 13–16). DAPI = diamidino-2-phenylindole.
Silencing PLC-β1 in the mPFC
To silence PLC-β1 specifically in the mPFC, we used lentivirus-mediated gene knockdown. First, we screened shPLC-β1 vectors for shRNA sequences that effectively knocked down PLC-β1 in C2C12 cell cultures (Fig. 1A). Eleven lentiviral vectors encoding different shPLC-β1 expression cassettes were validated for their ability to suppress PLC-β1 expression. Of these 11 vectors, shPLC-β1–11 most effectively reduced endogenous PLC-β1 expression in C2C12 cells (Fig. 1A, red rectangle). This vector (subsequently referred to simply as shPLC-β1) was used in all subsequent in vivo experiments. High-titre lentiviral vectors expressing shPLC-β1 were then prepared and bilaterally injected into the mPFC of 10-week-old wild-type mice; mice injected with shSCR vectors (expressing scrambled shRNA) were used as a control group (Fig. 1B, right panel). Because both constructs contain a green fluorescent protein reporter, we were able to determine infection efficiency and shRNA vector expression in individual brains in postmortem examinations conducted after behavioural studies by direct visualization with a fluorescence microscope (Fig. 1B, left panel).
To evaluate the effects of shPLC-β1 vectors on PLC-β1 expression in the brain, we performed immunohistochemical analyses using a PLC-β1 antibody. Injection of shSCR had no effect on PLC-β1 immunoreactivity, which was detected at high levels in the shSCR-injected mPFC (Fig. 1C, panels 1–8). In contrast, endogenous PLC-β1 expression was substantially decreased in the shPLC-β1-injected mPFC (Fig. 1C, panels 9–16). Infected cells labelled with DAPI showed no obvious neuronal injury, indicating that neither the lentiviral vector nor injection itself caused cytotoxicity (Fig. 1C, panels 7 and 15). In order to quantify gene silencing in the mPFC, we compared the percentage of PLC-β1-positive neurons in the shPLC-β1-injected mPFC with that in the shSCR-injected mPFC. We found that injection of the shPLC-β1 lentiviral vector induced a significant reduction (32.1% ± 2.5%) in the number of PLC-β1-positive neurons (p = 0.010; Fig. 1C, panel 13, and Fig. 2D).
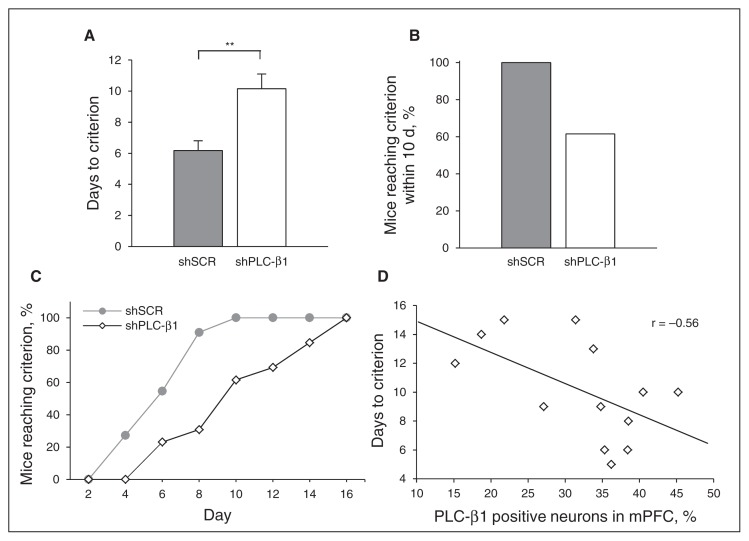
Fig. 2.
Impaired working memory in medial prefrontal cortext (mPFC)-specific phospholipase C-β1 (PLC-β1) knockdown mice. Performance of mice injected with small hairpin scrambled sequence RNA (shSCR) and shPLC-β1 in a T-maze-based delayed non-match-to-sample (DNMTS) task using a delay of 4 seconds. (A) Days to reach the criterion for shPLC-β1-injected (n = 13) and shSCR-injected (n = 11) mice (p = 0.003, 2-tailed t-test). (B) Number of mice reaching the criterion within 10 d, as a percentage. (C) Cumulative distribution of the number of mice reaching the test criterion as a function of the number of test days, as a percentage (p = 0.12, 2-sample Kolmogorov–Smirnov test). (D) Correlation between the percentage of PLC-β1-positive neurons in the mPFC (x axis) and the number of days to reach the DNMTS T-maze task criterion for shPLC-β1-injected mice (y axis; shPLC-β1: n = 13; Pearson correlation test, R = −0.56; p = 0.047, 2-tailed t test). Data are presented as means ± standard errors of the mean. *p < 0.05, **p < 0.01, 2-tailed t test.
Impaired working memory in mPFC-specific PLC-β1 knockdown mice
To investigate the role of mPFC-specific PLC-β1 in working memory, we administered the DNMTS T-maze task, which requires mice to integrate information (the sample run) with the learned rule (nonmatch to sample).33,34 In DNMTS T-maze tasks, shSCR-injected mice reached the criterion (correct choice on 11 of 12 consecutive trials, or 92%) within 6.18 ± 0.62 days, whereas shPLC-β1-injected mice needed significantly more trials to satisfy this criterion, requiring an average of 10.15 ± 0.95 days (p = 0.003, t test; Fig. 2A). All shSCR-injected mice reached the criterion within 10 days, but only 60% of shPLC-β1-injected mice reached this criterion within this period (Fig. 2B and C). Interestingly, a Pearson correlation analysis showed a significant negative correlation between the percentages of PLC-β1-positive neurons in the mPFC and the number of days required to reach the criterion of the DNMTS T-maze task in shPLC-β1-injected mice (Pearson R = −0.56, p = 0.047, t-test; Fig. 2D). On the other hand, there was no significant difference between shPLC-β1-injected mice and shSCR-injected mice in the time to reach the criterion in the simple right/left discrimination T-maze task (4.70 ± 0.83 d v. 5.43 ± 1.90; p = 0.30, t test; Appendix, Fig. S2A).34 Both shSCR- and shPLC-β1-injected mice reached the criterion within 10 days (Appendix, Fig. S2B). Collectively, these results indicate that PLC-β1 signalling in the mPFC is required for working memory.
Normal locomotor activity and anxiety in mPFC-specific PLC-β1 knockdown mice
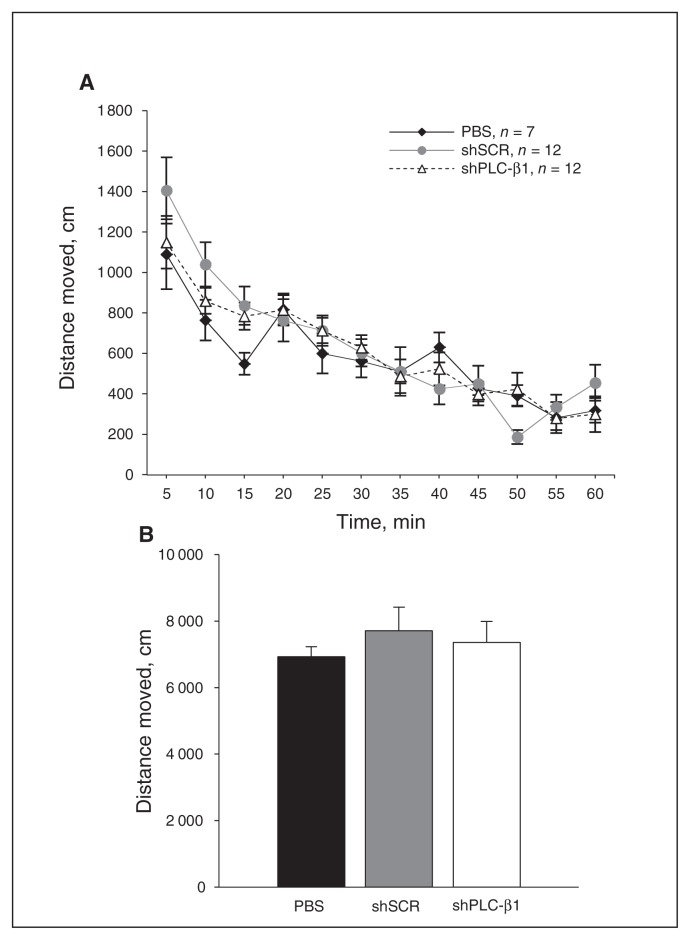
We next examined whether PLC-β1 deficiency in the mPFC is responsible for the hyperlocomotion and anxiolytic behaviours observed in PLC-β1−/− mice.10,12 In the open-field assay, shPLC-β1-injected mice showed no significant difference in the total amount of locomotor activity in the open field compared with shSCR- and vehicle (phosphate-buffered saline)–injected mice (F2,28 = 0.317, p = 0.73; Fig. 3A and B). Differences in anxiety levels could cause abnormal behaviours in cognitive tasks, including the DNMTS T-maze task. Accordingly, we tested shPLC-β1-injected mice in 3 anxiety behavioural assays: open field, light/dark box and elevated plus-maze. The percentages of distance and time spent in the centre of an open-field arena, quantified as described in the Methods section, did not differ significantly between shPLC-β1- and shSCR-injected mice (all p > 0.05; Appendix, Figs. S3A–D). In the light/dark box test, shPLC-β1-injected mice exhibited no significant difference in the number of transitions from the dark to the light compartment compared with shSCR-injected mice (p = 0.84; Appendix, Fig. S3E). Consistent with this observation, no significant differences in the time spent in the light chamber were observed between shPLC-β1- and shSCR-injected mice (p = 0.33; Appendix, Fig. S3F). Similarly, in the elevated plus-maze test, there was no significant difference in the percentage of entries into the aversive open arms (p = 0.30; Appendix, Fig. S3G) or the duration of time spent in open arms (p = 0.71; Appendix, Fig. S3H) between the 2 groups. These results indicate that PLC-β1 in the mPFC is not involved in locomotor activity or anxiety-related behaviours.
Fig. 3.
Normal locomotor activity in medial prefrontal cortext (mPFC)-specific phospholipase C-β1 (PLC-β1) knockdown mice. (A) Distance of spontaneous movement in an open-field for 1 hour was monitored at 5-minute intervals by digital video recording. (B) The small hairpin (sh)PLC-β1-injected mice show no significant difference in total distance of spontaneous locomotion compared with both scrambled sequence (shSCR)– and vehicle-injected mice (F2,28 = 0.317, p = 0.73). Data are presented as means ± standard errors of the mean (n = 12 per group).
Normal sensorimotor gating in mPFC-specific PLC-β1 knockdown mice
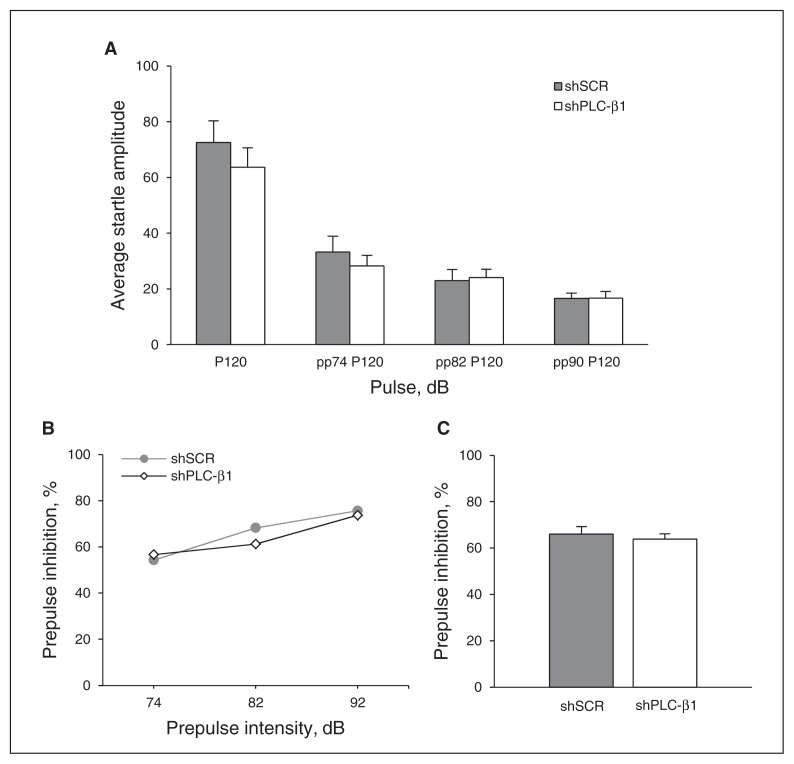
To examine whether decreased mPFC-specific PLC-β1 contributes to abnormal sensorimotor gating, we administered a PPI test, which measures the extent to which a weak prepulse stimulus attenuates the response to a subsequent startling stimulus. We measured the baseline ASR and then assessed the degree of PPI of the acoustic startle. No statistical difference was observed in the ASR to 120 dB between shSCR- and shPLC-β1-injected mice (p = 0.40, Fig. 4A). Startle responses to 120-dB pulses preceded by a prepulse of 3 different intensities (74, 82, 90 dB; F1,23 = 0.306, all p > 0.05; Fig. 4B) and average PPI values at all 3 prepulse intensities did not differ significantly between shSCR- and shPLC-β1-injected mice (all p > 0.05; Fig. 4C). These results indicate that PLC-β1 signalling in the mPFC is not related to the neural mechanism underlying PPI.
Fig. 4.
Normal sensorimotor gating in medial prefrontal cortex (mPFC)–specific phospholipase C-β1 (PLC-β1) knockdown mice. (A) Average startle amplitudes for various pulse types were not significantly different between small hairpin (sh)PLC-β1- and scrambled sequence (shSCR)–injected mice (shPLC-β1: n = 13, shSCR: n = 12; p > 0.05 for all pulse types, 2-tailed t test). (B) No significant difference in prepulse inhibition (PPI) percentage was observed between shSCR- and shPLC-β1-injected mice at 3 different prepulse intensities: 74, 82, 90 dB (shPLC-β1: n = 13, shSCR: n = 12; F1,23 = 0.306, p > 0.05 for all prepulse intensities). (C) The PPI values at all 3 prepulse intensities were lumped together and averaged. Data are presented as means ± standard errors of the mean. P = pulse; PP = prepulse.
Decreased sociability of PLC-β1−/− mice is not present in mPFC-specific PLC-β1-knockdown mice
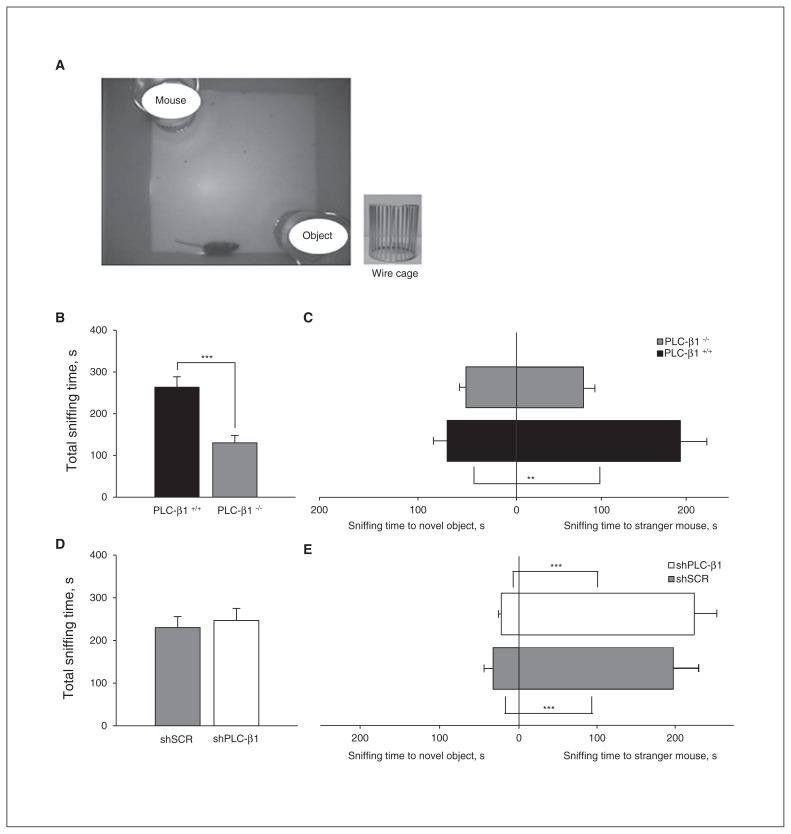
To investigate the role of PLC-β1 in social behaviours, we first tested PLC-β1−/− mice in a social behaviour apparatus (Fig. 5A).36 In this social behaviour assay, PLC-β1−/− mice showed a decrease in total sniffing time, which is the sum of time spent sniffing an unfamiliar wild-type mouse (stranger mouse) and nonsocial novel objects, than did PLC-β1+/+ mice (p < 0.001; Fig. 5B). These results indicate that the PLC-β1−/− mice exhibit decreased interest in novelty for a stranger mouse and inanimate objects compared with wild-type mice. Unlike PLC-β1+/+ mice, which spent more time sniffing the stranger mouse than nonsocial novel objects (F1,16 = 12.854, p = 0.002), PLC-β1−/− mice showed no difference in sniffing time between the stranger mouse and nonsocial novel objects (F1,20 = 3.351, p = 0.08; Fig. 5C). These results indicate that sociability, defined as the propensity to sniff a stranger mouse more than novel inanimate objects,36 is decreased in PLC-β1−/− mice compared with wild-type mice.
Fig. 5.
Social behaviour defects observed in phospholipase C-β1 (PLC-β1−/−) mice are not present in medial prefrontal cortex (mPFC)-specific PLC-β1 knockdown mice. (A) Social behaviour tests were performed in an open-field box (bottom) containing 2 wire cages (top), 1 enclosing a stranger mouse and the other containing novel objects. (B) Total sniffing time was reduced for PLC-β1−/− mice compared with PLC-β1+/+ mice (PLC-β1−/−: n = 11, PLC-β1+/+: n = 9; p < 0.001, 2-tailed t test). (C) The PLC-β1+/+ mice spent more time sniffing the stranger mouse than the novel objects (F1,16 = 12.854, p = 0.002). The PLC-β1−/− mice showed no difference in sniffing time between the stranger mouse and novel objects (F1,20 = 3.351, p = 0.08). (D) Small hairpin (sh)PLC-β1-injected mice showed no difference in total sniffing time compared with scrambled sequence (shSCR)-injected mice (shPLC-β1: n = 13, shSCR: n = 10; p = 0.68, 2-tailed t test). (E) The shPLC-β1-injected mice spent more time sniffing the stranger mouse than the novel objects (F1,24 = 48.306, p < 0.001), a behaviour similar to that of shSCR-injected mice (F1,18 = 23.050, p < 0.001). Data are presented as means ± standard errors of the mean. *p < 0.05, **p < 0.01, ***p < 0.001, 2-tailed t test and 1-way analysis of variance.
To determine whether the decreased interest in a stranger mouse or novel objects and the impaired sociability of PLC-β1−/− mice were attributable to a PLC-β1 defect in the mPFC, we conducted a social behaviour test in mPFC- specific PLC-β1 knockdown mice. The shPLC-β1-injected mice showed no difference in total sniffing time compared with shSCR-injected mice (p = 0.68; Fig. 5D). Moreover, shPLC-β1-injected mice spent more time sniffing the stranger mouse than the nonsocial novel objects (F1,24 = 48.306, p < 0.001); results were similar to those obtained with shSCR- injected mice (F1,18 = 23.050, p < 0.001; Fig. 5E). These results indicate that PLC-β1 is required for normal social behaviours, but mPFC-specific PLC-β1 signalling is not involved in regulating these behaviours.
Discussion
We have modelled the pathological relevance of the altered expression of PLC-β1 in the brains of patients with schizophrenia. We demonstrated that PLC-β1 knockdown in the mPFC of the mouse causes an impairment in working memory, one of the key symptoms of schizophrenia. This effect was specific to working memory; there were no effects of mPFC-specific PLC-β1 knockdown on other behaviours relevant to schizophrenic endophenotypes characteristic of PLC-β1−/− mice (e.g., locomotion, social behaviours, sensorimotor gating).
Previous reports have shown that cytotoxic lesions or acute inactivation of the mPFC in animals induces positive, negative and cognitive-like schizophrenia phenotypes, including a deficit of working memory.39,40 These observations suggest that deficits in the mPFC represent a key component of the pathophysiology in patients with schizophrenia.39,40 In addition, decreased PLC-β1 expression in the brains of patients with schizophrenia, particularly in the DLPFC, has suggested the possible pathogenic involvement of PLC-β1 in schizophrenia.13,14,21 Here, using mPFC-limited, shRNA-mediated silencing of PLC-β1, we generated a mouse model that mimics the decrease of PLC-β1 in the DLPFC of patients with schizophrenia. Behavioural characterization of these model mice revealed that mPFC-specific knockdown of PLC-β1 induced only the impaired working memory phenotype without producing the altered anxiety or other schizophrenia endophenotypes previously observed in PLC-β1−/− mice. Thus, our results suggest that the neural mechanisms underlying working memory in the mPFC may be different from those for anxiety or other schizophrenia endophenotypes of PLC-β1−/− mice. Moreover, our results indicate that the decrease of PLC-β1 expression in the DLPFC in patients with schizophrenia is a molecular change that is relevant to the working memory deficit in those patients.
Human behavioural and functional neuroimaging studies have described decreased activation in the DLPFC in patients with schizophrenia during working memory tasks and have demonstrated that stronger prefrontal-posterior parietal coupling predicts better working memory performance in patients with schizophrenia.41–43 In addition, abnormal brain oscillatory activity, recorded with electroencephalography (EEG) during working memory tests, has been reported in the frontal θ (4–8 Hz) and α (8–12 Hz) frequency bands of patients with schizophrenia.44,45 Recent human EEG studies have also detected abnormal circuitries involving the PFC and other brain regions, such as the temporal lobe and subcortical limbic structures, suggesting the importance of functional connectivity between the PFC and these other brain regions.46–48 Thus, alterations in synchronized brain oscillation reflect some of the neural changes that lead to schizophrenia.49,50
In mice, the mPFC receives indirect projections from the dorsal hippocampus (dHPC) and direct afferent inputs from the ventral hippocampus (vHPC).51,52 Furthermore, the mPFC has reciprocal connections with the amygdala and other subcortical limbic structures.53 θ-Frequency synchrony between the mPFC and the dHPC and/or β-frequency (13–30 Hz) synchrony between the mPFC and the mediodorsal thalamic nucleus are required for working memory.54,55 In addition, θ-frequency synchrony between the mPFC and the vHPC is important for anxiety and may suggest the involvement of the serotonergic (5-HT) system.56
Interestingly, our previous studies on PLC-β1−/− mice have shown that generation of cholinergic θ rhythm (4–8 Hz) in the hippocampus is mainly dependent on PLC-β1 signalling.9 The Gq-coupled muscarinic acetylcholine receptors (mAchRs), M1, M3 and M5, signal through PLC-β1.57 The M1 mAchRs are predominant in the cerebral cortex relative to other mAchR subtypes,58 and a decrease in M1 mAchRs in the cortex is observed in PLC-β1−/− mice.11 In this context, postmortem studies on the muscarinic system in schizophrenia using relatively subtype-selective radioligands have shown decreased receptor binding of the M1/M4-selective antagonist [3H] pirenzepine in the PFC of individuals with schizophrenia.59–61 Furthermore, a study on mPFC mAchRs in working memory previously reported that muscarinic transmission in the mPFC plays an important role in rodent working memory.62 Sabcomeline (CDD-0102A), a selective agonist of M1 mAchRs, enhances working memory in the mPFC, but not in the anterior cingulate cortex.63,64 Moreover, benzylquinolone carboxylic acid, a selective positive allosteric modulator of rat M1 mAchRs, has robust effects on M1-mediated responses in mPFC pyramidal cells, increases the firing of mPFC neurons in vivo, and improves mPFC-dependent forms of cognitive function.65 Thus, it is expected that modulation of cholinergic θ rhythms by the M1-PLC-β1 pathway in the mPFC may be involved in the functional connections among brain regions related to working memory. Although our current findings demonstrate the effect of mPFC PLC-β1 silencing in mice, additional studies designed to investigate G protein-coupled receptors linked to PLC-β1 in the mPFC will be necessary to fully elucidate the role of PLC-β1 signalling in the pathogenesis of schizophrenia.
Limitations
Other behavioural phenotypes of PLC-β1−/− mice, such as open-field, DNMTS T-maze, PPI, elevated plus-maze, and a light/dark transition task, have already been published in our previous studies,10,12 and these behavioural tests of mPFC-specific PLC-β1 knockdown mice were performed under the same experimental conditions as performed previously with PLC-β1−/− mice. On the other hand, the sociability test was not done for the PLC-β1−/−mice previously. Therefore, only social behavioural results of PLC-β1−/− mice and mPFC-specific PLC-β1 knockdown mice were included in the present study. Furthermore, from our present results, we infer that a similar process is happening in human schizophrenia. Nevertheless, we are aware of the possibility that the neural mechanisms underlying the working memory deficit in mice may be different from those in human schizophrenia. Understanding the pathophysiology for schizophrenia with our present study remains limited and raises more questions in this regard. We hope that these questions will be important for future in-depth studies, preferably in humans.
Conclusion
Our behavioural analysis of mPFC-specific PLC-β1 knockdown mice offers new insight into PLC-β1 hypofunction in the pathogenesis of schizophrenia and may further the understanding of neural mechanisms underlying working memory deficits in human schizophrenia.
Acknowledgements
We thank Dr. Taesup Cho and Dr. Sukchan Lee for his help in the preparation of the manuscripts. This work was supported by IBS-R001-D1, and the 21C Frontier Proteomics Program of the Ministry of Education, Sciecne and Technology, Korea.
Footnotes
Competing interests: None declared.
Contributors: S.-W. Kim, M. Seo and H.-S. Shin designed the study. S.-W. Kim, M. Seo, D.-S. Kim, M. Kang and Y.-S. Kim acquired the data, which S.-W. Kim, M. Seo, D.-S. Kim and H.-Y. Koh analyzed. H.-Y. Koh and H.-S. Shin reviewed the article, which all authors wrote and approved for publication.
References
- 1.Carter HR, Wallace MA, Fain JN. Activation of phospholipase C in rabbit brain membranes by carbachol in the presence of GTP gamma S; effects of biological detergents. Biochim Biophys Acta. 1990;1054:129–35. doi: 10.1016/0167-4889(90)90214-x. [DOI] [PubMed] [Google Scholar]
- 2.Sallés J, Wallace MA, Fain JN. Modulation of the phospholipase C activity in rat brain cortical membranes by simultaneous activation of distinct monoaminergic and cholinergic muscarinic receptors. Brain Res Mol Brain Res. 1993;20:111–7. doi: 10.1016/0169-328x(93)90115-6. [DOI] [PubMed] [Google Scholar]
- 3.Kim D, Jun KS, Lee SB, et al. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–3. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- 4.Hannan AJ, Blakemore C, Katsnelson A, et al. PLC-beta1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat Neurosci. 2001;4:282–8. doi: 10.1038/85132. [DOI] [PubMed] [Google Scholar]
- 5.Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 6.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–5. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe M, Nakamura M, Sato K, et al. Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cbeta in mouse brain. Eur J Neurosci. 1998;10:2016–25. doi: 10.1046/j.1460-9568.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukaya M, Uchigashima M, Nomura S, et al. Predominant expression of phospholipase Cbeta1 in telencephalic principal neurons and cerebellar interneurons, and its close association with related signaling molecules in somatodendritic neuronal elements. Eur J Neurosci. 2008;28:1744–59. doi: 10.1111/j.1460-9568.2008.06495.x. [DOI] [PubMed] [Google Scholar]
- 9.Shin J, Kim D, Bianchi R, et al. Genetic dissection of theta rhythm heterogeneity in mice. Proc Natl Acad Sci U S A. 2005;102:18165–70. doi: 10.1073/pnas.0505498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh HY, Kim D, Lee J, et al. Deficits in social behavior and sensorimotor gating in mice lacking phospholipase Cbeta1. Genes Brain Behav. 2008;7:120–8. doi: 10.1111/j.1601-183X.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 11.McOmish CE, Burrows E, Howard M, et al. Phospholipase C-beta1 knockout mice exhibit endophenotypes modeling schizophrenia which are rescued by environmental enrichment and clozapine administration. Mol Psychiatry. 2008;13:661–72. doi: 10.1038/sj.mp.4002046. [DOI] [PubMed] [Google Scholar]
- 12.McOmish CE, Burrows EL, Howard M, et al. PLC-beta1 knockout mice as a model of disrupted cortical development and plasticity: behavioral endophenotypes and dysregulation of RGS4 gene expression. Hippocampus. 2008;18:824–34. doi: 10.1002/hipo.20443. [DOI] [PubMed] [Google Scholar]
- 13.Lin XH, Kitamura N, Hashimoto T, et al. Opposite changes in phosphoinositide-specific phospholipase C immunoreactivity in the left prefrontal and superior temporal cortex of patients with chronic schizophrenia. Biol Psychiatry. 1999;46:1665–71. doi: 10.1016/s0006-3223(99)00036-0. [DOI] [PubMed] [Google Scholar]
- 14.Shirakawa O, Kitamura N, Lin XH, et al. Abnormal neurochemical asymmetry in the temporal lobe of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:867–77. doi: 10.1016/s0278-5846(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 15.Peruzzi D, Aluigi M, Manzoli L, et al. Molecular characterization of the human PLC beta1 gene. Biochim Biophys Acta. 2002;1584:46–54. doi: 10.1016/s1388-1981(02)00269-x. [DOI] [PubMed] [Google Scholar]
- 16.Arinami T, Ohtsuki T, Ishiguro H, et al. Genomewide high-density SNP linkage analysis of 236 Japanese families supports the existence of schizophrenia susceptibility loci on chromosomes 1p, 14q, and 20p. Am J Hum Genet. 2005;77:937–44. doi: 10.1086/498122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.du Bois TM, Deng C, Huang XF. Membrane phospholipid composition, alterations in neurotransmitter systems and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:878–88. doi: 10.1016/j.pnpbp.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Lo Vasco VR, Cardinale G, Polonia P. Deletion of PLCB1 gene in schizophrenia-affected patients. J Cell Mol Med. 2012;16:844–51. doi: 10.1111/j.1582-4934.2011.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346:477–81. doi: 10.1016/s0140-6736(95)91325-4. [DOI] [PubMed] [Google Scholar]
- 20.Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–34. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 21.Udawela M, Scarr E, Hannan AJ, et al. Phospholipase C beta 1 expression in the dorsolateral prefrontal cortex from patients with schizophrenia at different stages of illness. Aust N Z J Psychiatry. 2011;45:140–7. doi: 10.3109/00048674.2010.533364. [DOI] [PubMed] [Google Scholar]
- 22.Baddeley A. Working memory. Science. 1992;255:556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 23.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–57. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 24.Morris RG, Baddeley AD. Primary and working memory functioning in Alzheimer-type dementia. J Clin Exp Neuropsychol. 1988;10:279–96. doi: 10.1080/01688638808408242. [DOI] [PubMed] [Google Scholar]
- 25.Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, et al. Negative symptoms: a review of schizophrenia, melancholic depression and Parkinson’s disease. Brain Res Bull. 2006;70:312–21. doi: 10.1016/j.brainresbull.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Tek C, Gold J, Blaxton T, et al. Visual perceptual and working memory impairments in schizophrenia. Arch Gen Psychiatry. 2002;59:146–53. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- 27.Kyriakopoulos M, Dima D, Roiser JP, et al. Abnormal functional activation and connectivity in the working memory network in early-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2012;51:911–20.e2. doi: 10.1016/j.jaac.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49:1195–205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- 30.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–3. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 31.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Shin J, Gireesh G, Kim SW, et al. Phospholipase C beta 4 in the medial septum controls cholinergic theta oscillations and anxiety behaviors. J Neurosci. 2009;29:15375–85. doi: 10.1523/JNEUROSCI.3126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic- infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 2000;12:4457–66. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- 34.Kellendonk C, Simpson EH, Polan HJ, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–15. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Sakaue M, Ago Y, Baba A, et al. The 5-HT1A receptor agonist MKC-242 reverses isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2003;170:73–9. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- 36.Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 37.Crawley JN, Chen T, Puri A, et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–63. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Kim DS, Kim JE, Kwak SE, et al. Spatiotemporal characteristics of astroglial death in the rat hippocampo-entorhinal complex following pilocarpine-induced status epilepticus. J Comp Neurol. 2008;511:581–98. doi: 10.1002/cne.21851. [DOI] [PubMed] [Google Scholar]
- 39.Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–8. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 40.Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29:215–23. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan HY, Sust S, Buckholtz JW, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–77. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- 42.Van Snellenberg JX. Working memory and long-term memory deficits in schizophrenia: Is there a common substrate? Psychiatry Res. 2009;174:89–96. doi: 10.1016/j.pscychresns.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Henseler I, Falkai P, Gruber O. Disturbed functional connectivity within brain networks subserving domain-specific subcomponents of working memory in schizophrenia: relation to performance and clinical symptoms. J Psychiatr Res. 2010;44:364–72. doi: 10.1016/j.jpsychires.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Schmiedt C, Brand A, Hildebrandt H, et al. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25:936–47. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Bachman P, Kim J, Yee CM, et al. Abnormally high EEG alpha synchrony during working memory maintenance in twins discordant for schizophrenia. Schizophr Res. 2008;103:293–7. doi: 10.1016/j.schres.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ford JM, Mathalon DH, Whitfield S, et al. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–92. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 47.Lawrie SM, Buechel C, Whalley HC, et al. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–11. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 48.Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–86. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 49.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–65. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 50.Uhlhaas PJ, Roux F, Singer W, et al. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106:9866–71. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 52.Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro. Cereb Cortex. 2010;20:393–403. doi: 10.1093/cercor/bhp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 54.Sigurdsson T, Stark KL, Karayiorgou M, et al. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–7. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parnaudeau S, O’Neill PK, Bolkan SS, et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–62. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–69. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutkind JS, Novotny EA, Brann MR, et al. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci U S A. 1991;88:4703–7. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- 59.Dean B, Crook JM, Opeskin K, et al. The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol Psychiatry. 1996;1:54–8. [PubMed] [Google Scholar]
- 60.Crook JM, Tomaskovic-Crook E, Copolov DL, et al. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry. 2000;48:381–8. doi: 10.1016/s0006-3223(00)00918-5. [DOI] [PubMed] [Google Scholar]
- 61.Crook JM, Tomaskovic-Crook E, Copolov DL, et al. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry. 2001;158:918–25. doi: 10.1176/appi.ajp.158.6.918. [DOI] [PubMed] [Google Scholar]
- 62.Ragozzino ME, Kesner RP. The effects of muscarinic cholinergic receptor blockade in the rat anterior cingulate and prelimbic/infralimbic cortices on spatial working memory. Neurobiol Learn Mem. 1998;69:241–57. doi: 10.1006/nlme.1998.3823. [DOI] [PubMed] [Google Scholar]
- 63.Hatcher JP, Loudon JM, Hagan JJ, et al. Sabcomeline (SB-202026), a functionally selective M1 receptor partial agonist, reverses delay-induced deficits in the T-maze. Psychopharmacology (Berl) 1998;138:275–82. doi: 10.1007/s002130050672. [DOI] [PubMed] [Google Scholar]
- 64.Ragozzino ME, Artis S, Singh A, et al. The selective M1 muscarinic cholinergic agonist CDD-0102A enhances working memory and cognitive flexibility. J Pharmacol Exp Ther. 2012;340:588–94. doi: 10.1124/jpet.111.187625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shirey JK, Brady AE, Jones PJ, et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–86. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]