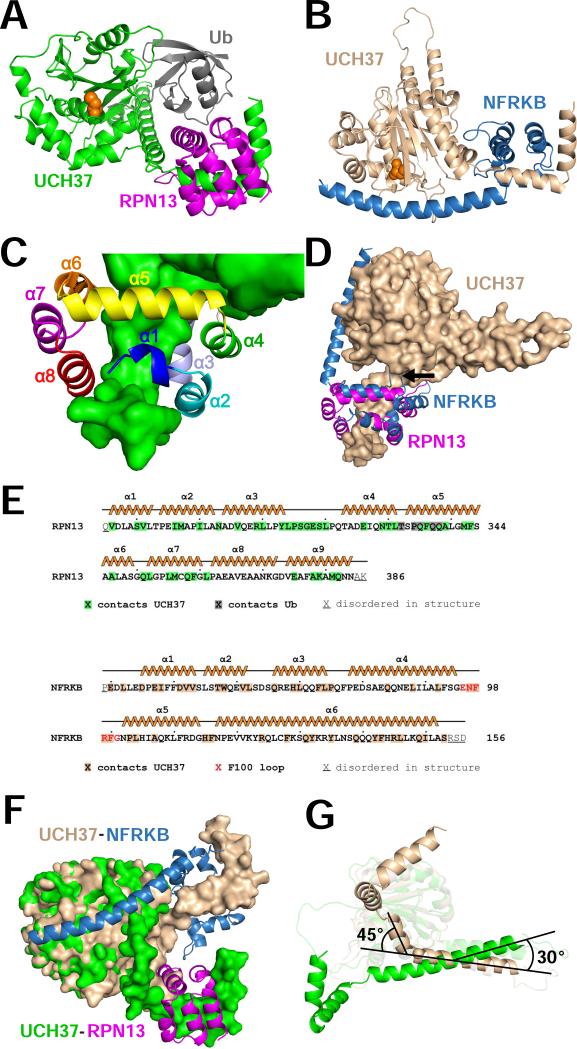
Figure 2. Structures of RPN13 and NFRKB complexes.
(A) Overview of the RPN13-UCH37-ubiquitin complex.
(B) Overview of the NFRKB-UCH37 complex. Panels A and B show the same relative orientation for UCH37 residues from the last two turns of α9 to the end of α11.
(C) RPN13 with helices labeled.
(D) Superposition of RPN13 and NFRKB following alignment of the C-terminal residues of UCH37 α9, α10, and α11. The F100 loop of NFRKB is indicated by an arrow.
(E) RPN13 and NFRKB sequences and secondary structures. All residues in the crystallized constructs are indicated.
(F) Surface representation of RPN13CTD and NFRKBNTD complexes overlapped on the catalytic UCH domain. RPN13CTD and NFRKBNTD shown as ribbons. For clarity, ubiquitin is not shown.
(G) Ribbon representation, viewed orthogonally to panel A. The 30° tilt of α8 and α9 and the 45° kink in α9 are indicated. RPN13 and NFRKB are not shown. R279 CA is indicated with a sphere.