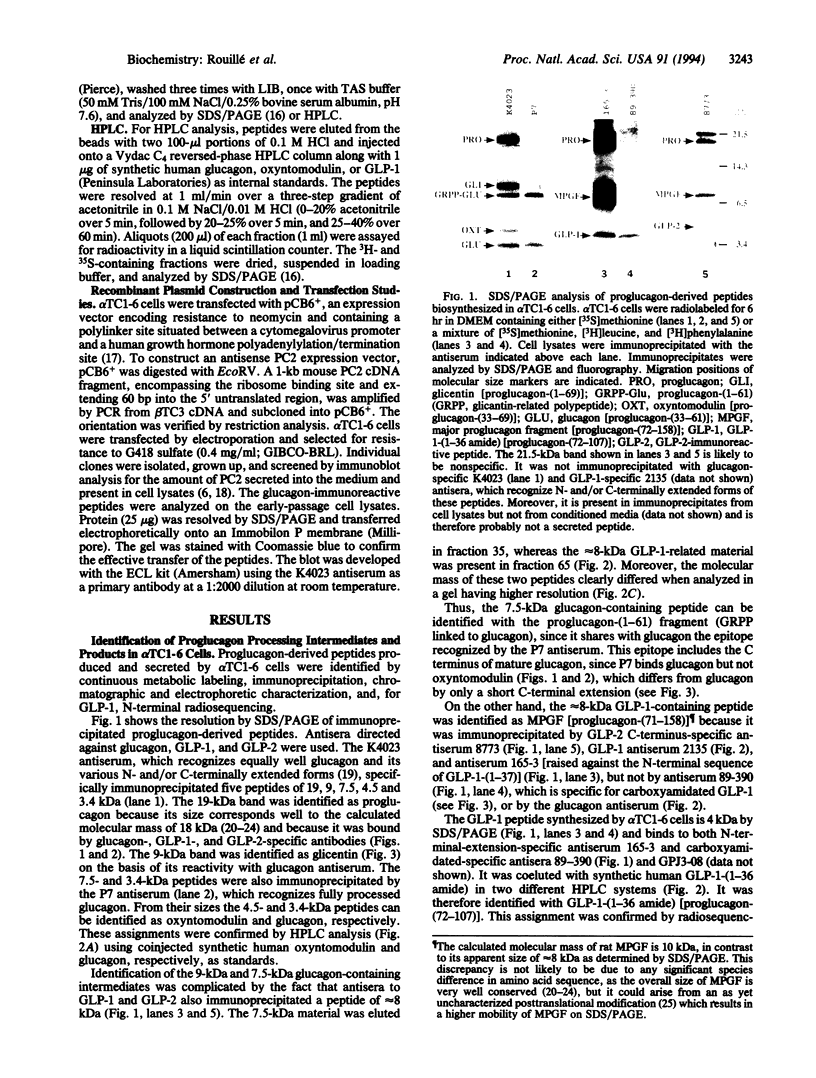
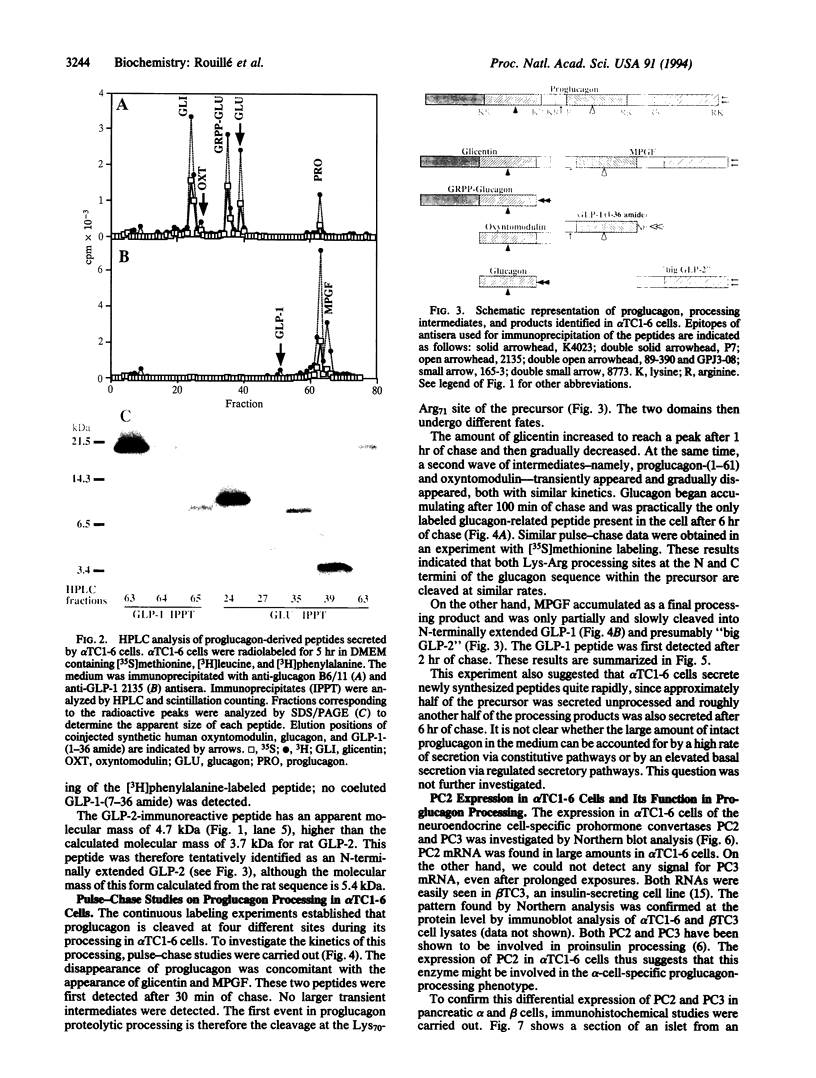
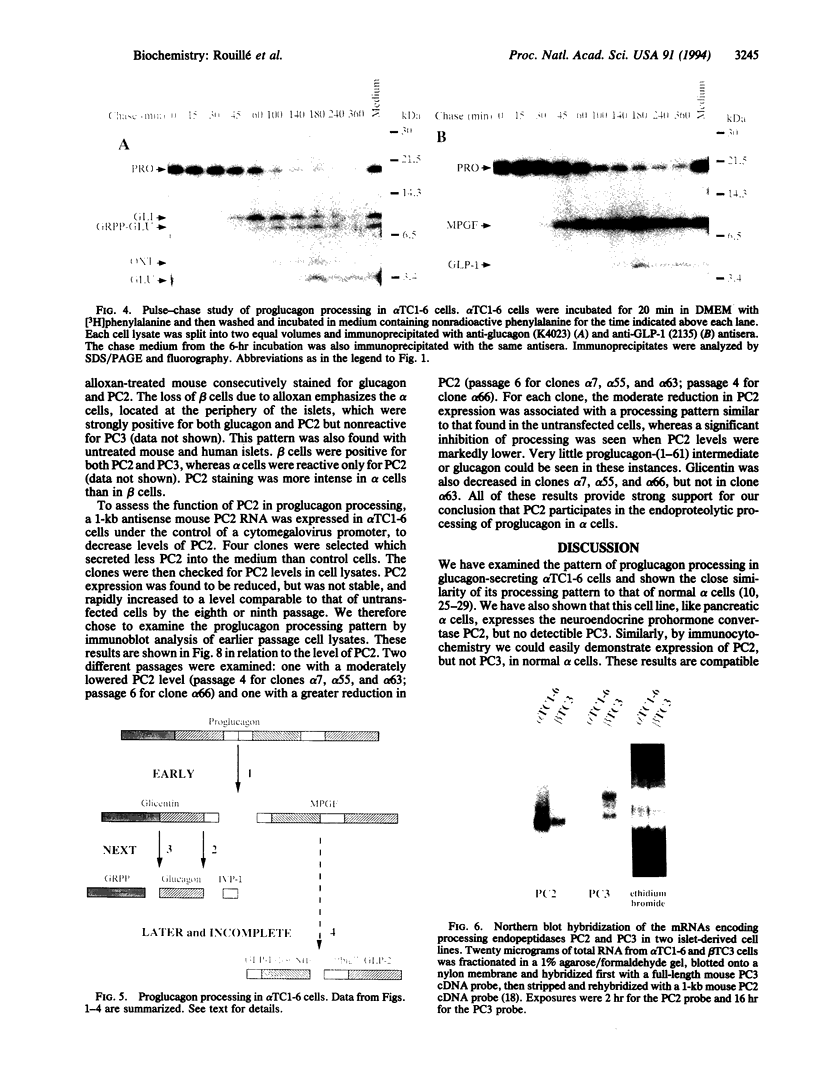
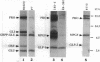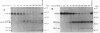Abstract
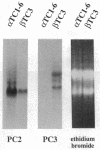
Proglucagon is processed differentially in the pancreatic alpha cells and the intestinal L cells to yield either glucagon or glucagon-like peptide 1, respectively, structurally related hormones with opposing metabolic actions. Here, we have studied the processing of proglucagon in alpha TC1-6 cells, an islet-cell line transformed by simian virus 40 large tumor (T) antigen, a model of the pancreatic alpha cell. We found that these cells process proglucagon at certain dibasic cleavage sites to release glucagon and only small amounts of glucagon-like peptide 1, as demonstrated by both continuous and pulse-chase labeling experiments. Both normal islet alpha cells and alpha TC1-6 cells were shown to express the prohormone convertase PC2 at high levels, but not the related protease PC3. Expression of PC2 antisense RNA in alpha TC1-6 cells inhibited both PC2 production and proglucagon processing concomitantly. We conclude that PC2 is the key endoprotease responsible for proglucagon processing in cells with the alpha-cell phenotype.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C. Exon duplication and divergence in the human preproglucagon gene. 1983 Jul 28-Aug 3Nature. 304(5924):368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Santerre R. F., Mullenbach G. T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983 Apr 21;302(5910):716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I. The glucagon superfamily: precursor structure and gene organization. Peptides. 1986;7 (Suppl 1):27–36. doi: 10.1016/0196-9781(86)90160-9. [DOI] [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Day R., Chrétien M., Seidah N. G. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist B. T., Eipper B. A., Mains R. E. Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol. 1991 Dec;5(12):2014–2024. doi: 10.1210/mend-5-12-2014. [DOI] [PubMed] [Google Scholar]
- Drucker D. J., Mojsov S., Habener J. F. Cell-specific post-translational processing of preproglucagon expressed from a metallothionein-glucagon fusion gene. J Biol Chem. 1986 Jul 25;261(21):9637–9643. [PubMed] [Google Scholar]
- Efrat S., Linde S., Kofod H., Spector D., Delannoy M., Grant S., Hanahan D., Baekkeskov S. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi K., Leiter E. H. Comparison of cytokine effects on mouse pancreatic alpha-cell and beta-cell lines. Viability, secretory function, and MHC antigen expression. Diabetes. 1990 Apr;39(4):415–425. doi: 10.2337/diab.39.4.415. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of pancreatic and gut glucagon in plasma. Diabetologia. 1971 Feb;7(1):10–19. doi: 10.1007/BF02346248. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Gros P., Habener J. F. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J Biol Chem. 1984 Nov 25;259(22):14082–14087. [PubMed] [Google Scholar]
- Hirota M., Shimizu I., Ohboshi C., Nishino T., Shima K. A large molecular form of glucagon-like peptide-1 (GLP-1) immunoreactivity is co-released with glucagon from pancreas by arginine in normal subjects. Clin Chim Acta. 1987 Aug 31;167(3):293–302. doi: 10.1016/0009-8981(87)90349-4. [DOI] [PubMed] [Google Scholar]
- Lopez L. C., Frazier M. L., Su C. J., Kumar A., Saunders G. F. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5485–5489. doi: 10.1073/pnas.80.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott D., Gillece-Castro B., Gorman C. M. Prohormone convertase-1 will process prorelaxin, a member of the insulin family of hormones. Mol Endocrinol. 1992 Sep;6(9):1441–1450. doi: 10.1210/mend.6.9.1435788. [DOI] [PubMed] [Google Scholar]
- Mojsov S., Heinrich G., Wilson I. B., Ravazzola M., Orci L., Habener J. F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986 Sep 5;261(25):11880–11889. [PubMed] [Google Scholar]
- Nakayama K., Hosaka M., Hatsuzawa K., Murakami K. Cloning and functional expression of a novel endoprotease involved in prohormone processing at dibasic sites. J Biochem. 1991 Jun;109(6):803–806. doi: 10.1093/oxfordjournals.jbchem.a123461. [DOI] [PubMed] [Google Scholar]
- Orskov C., Bersani M., Johnsen A. H., Højrup P., Holst J. J. Complete sequences of glucagon-like peptide-1 from human and pig small intestine. J Biol Chem. 1989 Aug 5;264(22):12826–12829. [PubMed] [Google Scholar]
- Orskov C. Glucagon-like peptide-1, a new hormone of the entero-insular axis. Diabetologia. 1992 Aug;35(8):701–711. [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Knuhtsen S., Baldissera F. G., Poulsen S. S., Nielsen O. V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986 Oct;119(4):1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Schiltz E. Conversion of proglucagon in pancreatic alpha cells: the major endproducts are glucagon and a single peptide, the major proglucagon fragment, that contains two glucagon-like sequences. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5007–5011. doi: 10.1073/pnas.81.16.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C., Tager H. S., Carroll R. J., Steiner D. F. Identification and processing of proglucagon in pancreatic islets. Nature. 1979 Nov 15;282(5736):260–266. doi: 10.1038/282260a0. [DOI] [PubMed] [Google Scholar]
- Powers A. C., Efrat S., Mojsov S., Spector D., Habener J. F., Hanahan D. Proglucagon processing similar to normal islets in pancreatic alpha-like cell line derived from transgenic mouse tumor. Diabetes. 1990 Apr;39(4):406–414. doi: 10.2337/diab.39.4.406. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seino S., Welsh M., Bell G. I., Chan S. J., Steiner D. F. Mutations in the guinea pig preproglucagon gene are restricted to a specific portion of the prohormone sequence. FEBS Lett. 1986 Jul 14;203(1):25–30. doi: 10.1016/0014-5793(86)81429-6. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Montag A. G., Thomas G., Albiges-Rizo C., Carroll R., Benig M., Phillips L. A., Martin S., Ohagi S., Gardner P. Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8822–8826. doi: 10.1073/pnas.89.18.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Stout J. T., Caskey C. T. Antisense RNA inhibition of HPRT synthesis. Somat Cell Mol Genet. 1990 Jul;16(4):369–382. doi: 10.1007/BF01232465. [DOI] [PubMed] [Google Scholar]
- Thomas L., Leduc R., Thorne B. A., Smeekens S. P., Steiner D. F., Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]