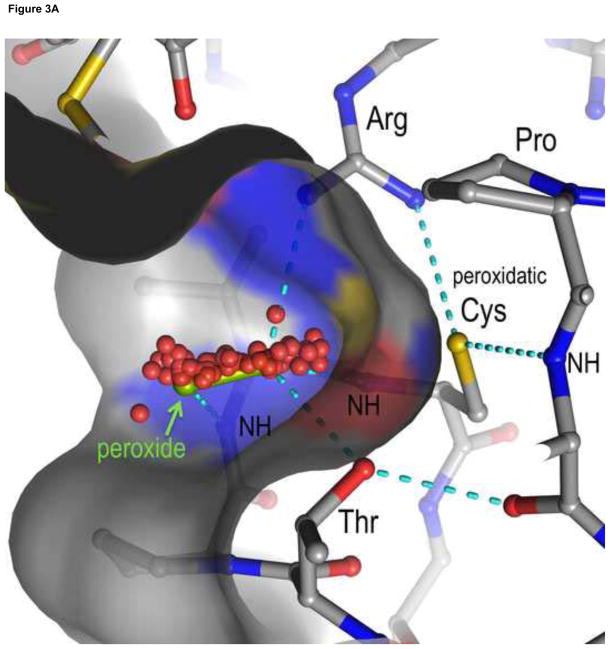
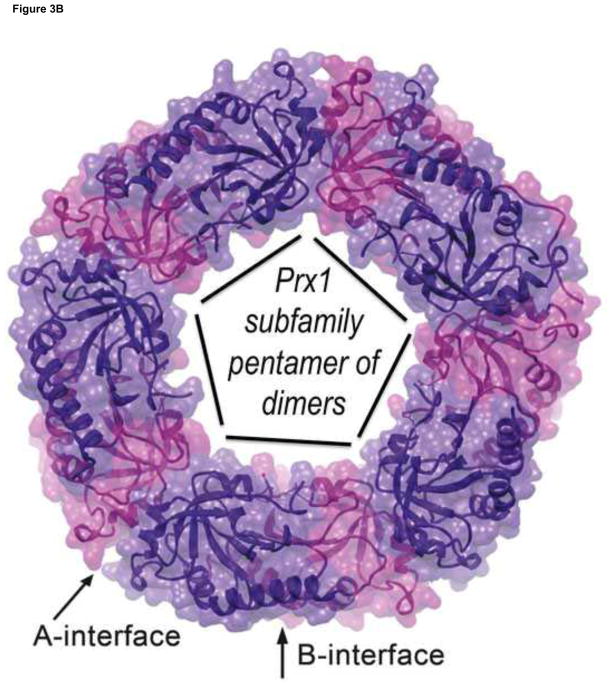
Figure 3.
Views of the Prx active site and Prx1 subfamily decamer. A. The fully-folded active site pocket of a representative Prx is shown with the molecular surface (with grey carbons, red oxygens, blue nitrogens and yellow sulfurs. In the substrate binding pocket is shown an H2O2 molecule (green) as seen in the Michaelis complex of an Aeropyrum pernix Prx [17] and hydrogen bonds (cyan dashed lines) from backbone atoms and the conserved Thr and Arg side chains that stabilize the CP residue and substrate. Also shown are dozens of oxygens (red) representing the positions of water molecules that are bound to various un-liganded FF actives sites (gathered as described in [18]). The water positions clearly define a track along which the substrate oxygens will be stabilized as they move during the reaction. B. Decamer commonly seen for Prx1 subfamily enzymes such as mammalian PrxI, PrxII, PrxIII and PrxIV. Emphasized is how it is constructed of a pentamer of B-type dimers associating through the A-type interfaces.