Figure 3.
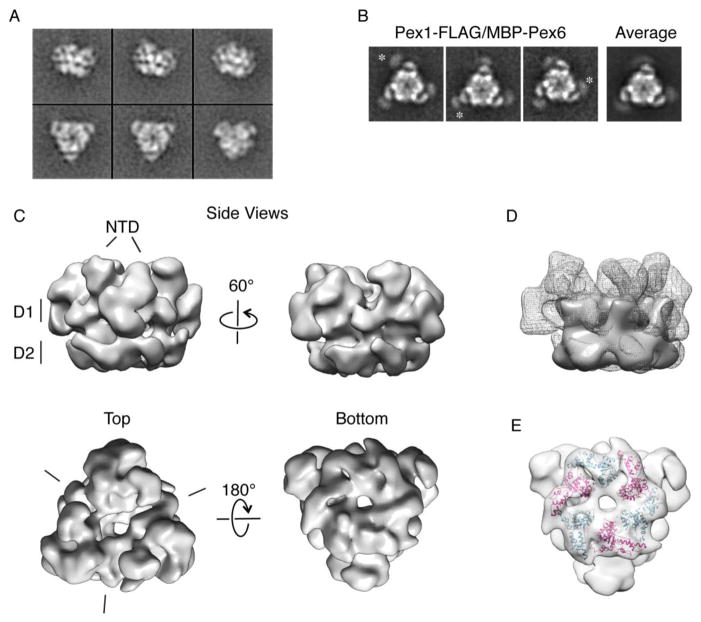
A) 2D class averages from negative-stain electron microscopy of recombinant Pex1-FLAG/His-Pex6 in the presence of 3 mM ATP.
B) Three representative class averages of Pex1-FLAG/MBP-Pex6 show extra density for MBP at the apices of the heterohexamer, consistent with an attachment to the extended N-terminal domain. 2D classes were aligned and averaged to show the flexible attachment of the MBP tag to the N-terminal extensions of Pex6 in the heterohexamer.
C) The 3D reconstruction of the Pex1-FLAG/His-Pex6 heterohexamer at 23-Å resolution. D1 and D2 mark the individual ATPase rings. The top view depicts the D1 ring and NTDs (left), and the bottom view shows the D2 ring (right). In the top view, the hash marks delineate the “dimer” interfaces in the “trimer of dimer” arrangement.
D) The density of truncated NSF (EMDB: 2041) constituting only the D1 and D2 domains was docked into the 3D reconstruction of Pex1/Pex6 to emphasize the density of the Pex1/Pex6 N-terminal domains.
E) The Pex1/Pex6 D2 ATPase ring exhibits the canonical architecture of the small α-helical AAA+ subdomain interacting with the large AAA+ subdomain of the counter-clockwise-neighboring subunit. Shown are homology models for the Pex1 and Pex6 D2 domains docked into the density of the Pex1/Pex6 D2 ATPase ring.