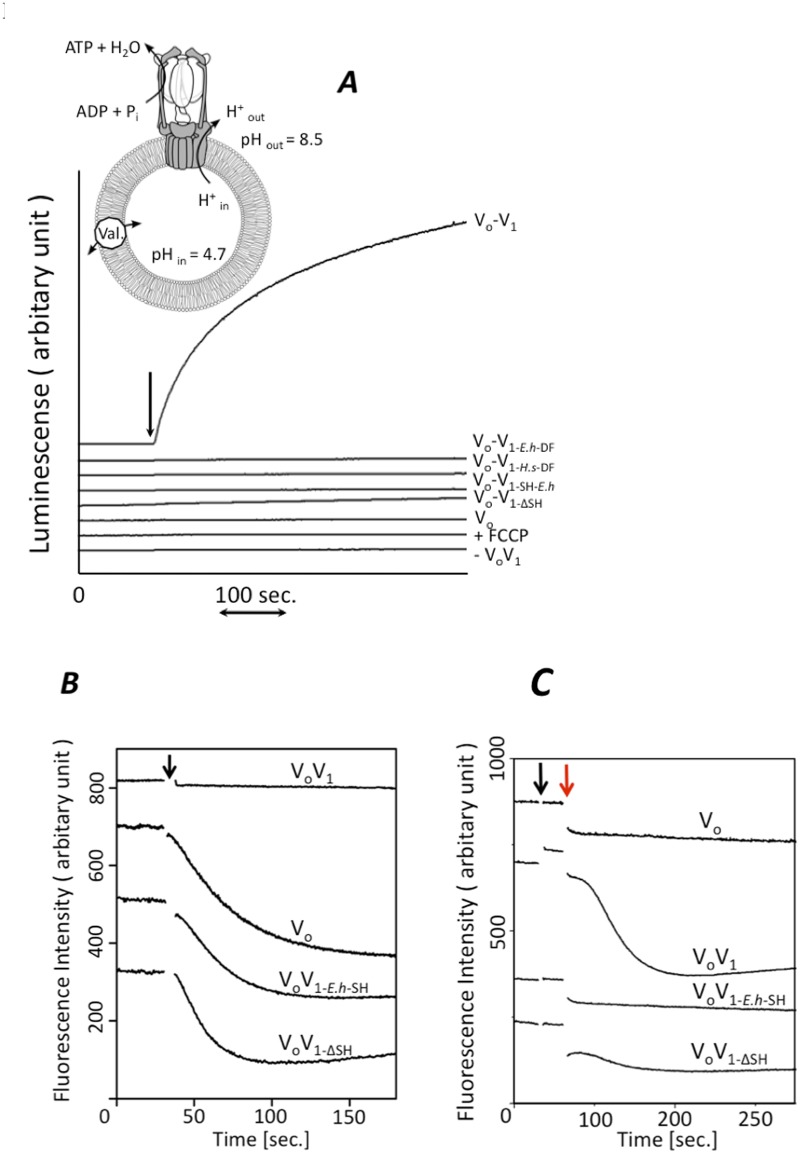
Fig 4. Function of the short helix of V1-D subunit for energy coupling.
A, Analysis of ATP synthesis by the reconstituted complexes. inset; schematic model of the experimental system [16]. The reconstituted VoV1 was incorporated into liposomes, then energized by an acid base transition procedure described in the Materials and Methods. Each line shows the raw data for ATP synthesis by each reconstituted complex at a Δ pH of 3.8. The reactions were initiated by addition of acidified proteoliposomes into the base buffer, as indicated by the arrow. Final concentrations were 2 μg/ml of VoV1 and 1 mM ADP. The synthesized ATP was monitored by the luciferin-luciferase assay [18]. Analysis of proton channel (B) and proton pump activity (C) coupled with ATP hydrolysis by proteoliposomes. K+-loaded proteoliposomes containing the reconstituted complexes were prepared as described in the Materials and Methods. Proton influx was initiated by addition of 20 ng of valinomycin at the time indicated by the black arrow (B) and proton pump was initiated by addition of ATP-Mg at finally 1mM followed by the addition of valinomycin at the time indicated by the red arrow (C). The ACMA fluorescence emission (480nm) was recorded at 25°C.