Figure 2. Combined Notch inhibition in T cells and CD8 depletion markedly delays heart allograft rejection and decreases the number of cytokine-producing donor-reactive T cells.
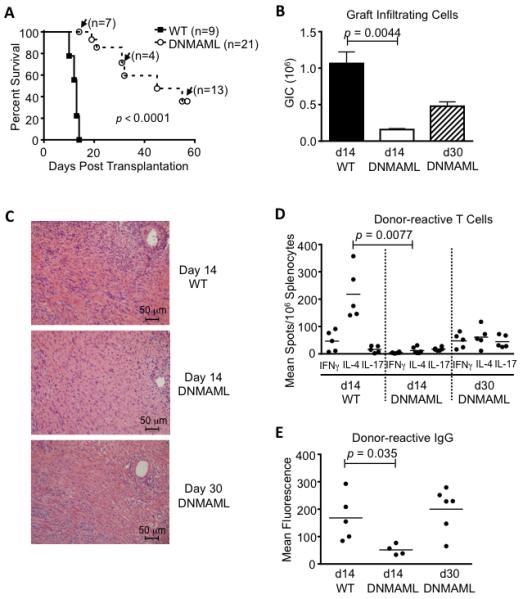
(A) After antibody-mediated CD8 depletion, vascularized cardiac BALB/c allografts were established in wild-type C57BL/6 recipients (WT, n=9) or in C57BL/6 mice expressing DNMAML in mature T cells (DNMAML, n=21). Survival of the heart allografts was markedly prolonged in DNMAML recipients (p<0.0001). Arrows indicate functioning grafts that were harvested at defined time points before rejection for preplanned immunological analysis (with subsequent graft survival data censored); (B) Decreased numbers of graft-infiltrating cells at day 14 in DNMAML recipients. Slightly higher numbers were recovered subsequently at day 30 (d14 WT: n=9; d14 DNMAML n=7; d30 DNMAML n=6); (C) Histology showing cellular infiltrates in the rejected hearts at day 14 or day 30; (D) Enumeration of donor-reactive cells producing IFNγ, IL-4 and IL-17 in the spleens of transplant recipients. IL-4 production dominates in the CD8-depleted model (43) and was markedly decreased in DNMAML recipients (p=0.0077); (E) Donor-reactive serum IgG, showing decreased levels at day 14 in DNMAML recipients (p=0.0351), but subsequent rise at day 30.
